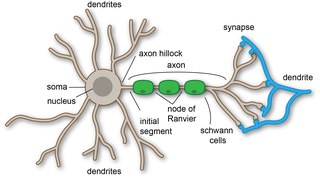
A dendrite or dendron is a branched protoplasmic extension of a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

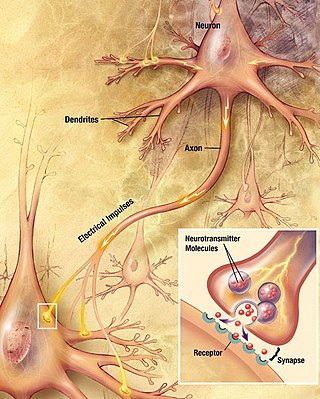
Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses - specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. Non-animals like plants and fungi do not have nerve cells.
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and in some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.

An electrical synapse is a mechanical and electrically conductive link between two neighboring neurons that is formed at a narrow gap between the pre- and postsynaptic neurons known as a gap junction. At gap junctions, such cells approach within about 3.8 nm of each other, a much shorter distance than the 20- to 40-nanometer distance that separates cells at chemical synapse. In many animals, electrical synapse-based systems co-exist with chemical synapses.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. IPSPs were first investigated in motorneurons by David P. C. Lloyd, John Eccles and Rodolfo Llinás in the 1950s and 1960s. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell to cell signalling. Inhibitory presynaptic neurons release neurotransmitters that then bind to the postsynaptic receptors; this induces a change in the permeability of the postsynaptic neuronal membrane to particular ions. An electric current that changes the postsynaptic membrane potential to create a more negative postsynaptic potential is generated, i.e. the postsynaptic membrane potential becomes more negative than the resting membrane potential, and this is called hyperpolarisation. To generate an action potential, the postsynaptic membrane must depolarize—the membrane potential must reach a voltage threshold more positive than the resting membrane potential. Therefore, hyperpolarisation of the postsynaptic membrane makes it less likely for depolarisation to sufficiently occur to generate an action potential in the postsynaptic neurone.

Rodolfo Llinás Riascos is a Colombian and American neuroscientist. He is currently the Thomas and Suzanne Murphy Professor of Neuroscience and Chairman Emeritus of the Department of Physiology & Neuroscience at the NYU School of Medicine. Llinás has published over 800 scientific articles.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.

In neuroscience, Golgi cells are inhibitory interneurons found within the granular layer of the cerebellum. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network, where the inhibitory interneuron was identified anatomically. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

Neurotransmission is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron, and bind to and react with the receptors on the dendrites of another neuron a short distance away. A similar process occurs in retrograde neurotransmission, where the dendrites of the postsynaptic neuron release retrograde neurotransmitters that signal through receptors that are located on the axon terminal of the presynaptic neuron, mainly at GABAergic and glutamatergic synapses.
Neural facilitation, also known as paired-pulse facilitation (PPF), is a phenomenon in neuroscience in which postsynaptic potentials (PSPs) evoked by an impulse are increased when that impulse closely follows a prior impulse. PPF is thus a form of short-term synaptic plasticity. The mechanisms underlying neural facilitation are exclusively pre-synaptic; broadly speaking, PPF arises due to increased presynaptic Ca2+
concentration leading to a greater release of neurotransmitter-containing synaptic vesicles. Neural facilitation may be involved in several neuronal tasks, including simple learning, information processing, and sound-source localization.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

The Calyx of Held is a particularly large synapse in the mammalian auditory central nervous system, so named after Hans Held who first described it in his 1893 article Die centrale Gehörleitung because of its resemblance to the calyx of a flower. Globular bushy cells in the anteroventral cochlear nucleus (AVCN) send axons to the contralateral medial nucleus of the trapezoid body (MNTB), where they synapse via these calyces on MNTB principal cells. These principal cells then project to the ipsilateral lateral superior olive (LSO), where they inhibit postsynaptic neurons and provide a basis for interaural level detection (ILD), required for high frequency sound localization. This synapse has been described as the largest in the brain.

Axon terminals are distal terminations of the telodendria (branches) of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell, or neuron, that conducts electrical impulses called action potentials away from the neuron's cell body, or soma, in order to transmit those impulses to other neurons, muscle cells or glands.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.

The active zone or synaptic active zone is a term first used by Couteaux and Pecot-Dechavassinein in 1970 to define the site of neurotransmitter release. Two neurons make near contact through structures called synapses allowing them to communicate with each other. As shown in the adjacent diagram, a synapse consists of the presynaptic bouton of one neuron which stores vesicles containing neurotransmitter, and a second, postsynaptic neuron which bears receptors for the neurotransmitter, together with a gap between the two called the synaptic cleft. When an action potential reaches the presynaptic bouton, the contents of the vesicles are released into the synaptic cleft and the released neurotransmitter travels across the cleft to the postsynaptic neuron and activates the receptors on the postsynaptic membrane.
Neurotransmitters are released into a synapse in packaged vesicles called quanta. One quantum generates a miniature end plate potential (MEPP) which is the smallest amount of stimulation that one neuron can send to another neuron. Quantal release is the mechanism by which most traditional endogenous neurotransmitters are transmitted throughout the body. The aggregate sum of many MEPPs is an end plate potential (EPP). A normal end plate potential usually causes the postsynaptic neuron to reach its threshold of excitation and elicit an action potential. Electrical synapses do not use quantal neurotransmitter release and instead use gap junctions between neurons to send current flows between neurons. The goal of any synapse is to produce either an excitatory postsynaptic potential (EPSP) or an inhibitory postsynaptic potential (IPSP), which generate or repress the expression, respectively, of an action potential in the postsynaptic neuron. It is estimated that an action potential will trigger the release of approximately 20% of an axon terminal's neurotransmitter load.
Dendrodendritic synapses are connections between the dendrites of two different neurons. This is in contrast to the more common axodendritic synapse (chemical synapse) where the axon sends signals and the dendrite receives them. Dendrodendritic synapses are activated in a similar fashion to axodendritic synapses in respects to using a chemical synapse. An incoming action potential permits the release of neurotransmitters to propagate the signal to the post synaptic cell. There is evidence that these synapses are bi-directional, in that either dendrite can signal at that synapse. Ordinarily, one of the dendrites will display inhibitory effects while the other will display excitatory effects. The actual signaling mechanism utilizes Na+ and Ca2+ pumps in a similar manner to those found in axodendritic synapses.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.