Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. Thus, the longer the biological half-life of a toxic substance, the greater the risk of chronic poisoning, even if environmental levels of the toxin are not very high. Bioaccumulation, for example in fish, can be predicted by models. Hypothesis for molecular size cutoff criteria for use as bioaccumulation potential indicators are not supported by data. Biotransformation can strongly modify bioaccumulation of chemicals in an organism.
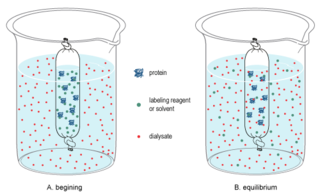
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.

A leachate is any liquid that, in the course of passing through matter, extracts soluble or suspended solids, or any other component of the material through which it has passed.

Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of chemicals, and separation of substances.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
Electrocoagulation (EC) is a technique used for wastewater treatment, wash water treatment, industrially processed water, and medical treatment. Electrocoagulation has become a rapidly growing area of wastewater treatment due to its ability to remove contaminants that are generally more difficult to remove by filtration or chemical treatment systems, such as emulsified oil, total petroleum hydrocarbons, refractory organics, suspended solids, and heavy metals. There are many brands of electrocoagulation devices available, and they can range in complexity from a simple anode and cathode to much more complex devices with control over electrode potentials, passivation, anode consumption, cell REDOX potentials as well as the introduction of ultrasonic sound, ultraviolet light and a range of gases and reactants to achieve so-called Advanced Oxidation Processes for refractory or recalcitrant organic substances.
Environmental monitoring is the processes and activities that are done to characterize and describe the state of the environment. It is used in the preparation of environmental impact assessments, and in many circumstances in which human activities may cause harmful effects on the natural environment. Monitoring strategies and programs are generally designed to establish the current status of an environment or to establish a baseline and trends in environmental parameters. The results of monitoring are usually reviewed, analyzed statistically, and published. A monitoring program is designed around the intended use of the data before monitoring starts.
Biosorption is a physiochemical process that occurs naturally in certain biomass which allows it to passively concentrate and bind contaminants onto its cellular structure. Biosorption can be defined as the ability of biological materials to accumulate heavy metals from wastewater through metabolically mediated or physico-chemical pathways of uptake. Though using biomass in environmental cleanup has been in practice for a while, scientists and engineers are hoping this phenomenon will provide an economical alternative for removing toxic heavy metals from industrial wastewater and aid in environmental remediation.
Chemcatcher is a passive sampling device for monitoring a variety of pollutants in water. It is a reusable three component, water-tight PTFE body. Two different designs are available to accommodate different types of commercially available 47 mm diameter receiving phase disks.
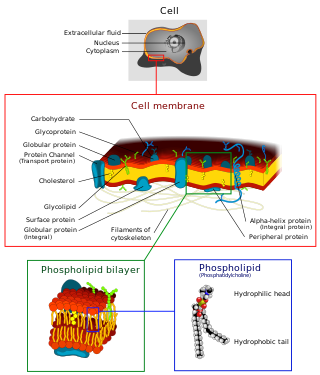
The cell membrane is a biological membrane that separates and protects the interior of a cell from the outside environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose.
Phytoextraction is a subprocess of phytoremediation in which plants remove dangerous elements or compounds from soil or water, most usually heavy metals, metals that have a high density and may be toxic to organisms even at relatively low concentrations. The heavy metals that plants extract are toxic to the plants as well, and the plants used for phytoextraction are known hyperaccumulators that sequester extremely large amounts of heavy metals in their tissues. Phytoextraction can also be performed by plants that uptake lower levels of pollutants, but due to their high growth rate and biomass production, may remove a considerable amount of contaminants from the soil.
Simultaneously extracted metals/Acid-volatile sulfide (SEM-AVS) is an approach used in the field of aquatic toxicology to assess the potential for metal ions found in sediment to cause toxic effects in organisms dwelling in the sediment. In this approach, the amounts of several heavy metals in a sediment sample are measured in a laboratory; at the same time, the amount of acid-volatile sulfide is determined. Based on the chemical interactions between heavy metals (SEM) and acid-volatile sulfide (AVS), the concentrations of these two components can be used to assess the potential for toxicity to sediment-dwelling organisms.
A polar organic chemical integrative sampler (POCIS) is a passive sampling device which allows for the in situ collection of a time-integrated average of hydrophilic organic contaminants developed by researchers with the United States Geological Survey in Columbia, Missouri. POCIS provides a means for estimating the toxicological significance of waterborne contaminants. The POCIS sampler mimics the respiratory exposure of organisms living in the aquatic environment and can provide an understanding of bioavailable contaminants present in the system. POCIS can be deployed in a wide range of aquatic environments and is commonly used to assist in environmental monitoring studies.

Microtox is an in vitro testing system which uses bioluminescent bacteria to detect toxic substances in different substrates such as water, air, soils and sediments. Allivibrio fischeri are non-pathogenic, marine, bacteria that luminesce as a natural part of their metabolism. When exposed to a toxic substance, the respiratory process of the bacteria is disrupted, reducing light output. Allivibrio fischeri have demonstrated high sensitivity across a wide variety of toxic substances. Response to toxicity is observed as a change in luminescence, which is a by-product of cellular respiration. This change can be used to calculate a percent inhibition of Allivibrio fischeri that directly correlates to toxicity.
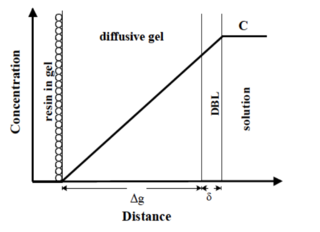
The diffusive gradients in thin films (DGT) technique is an environmental chemistry technique for the detection of elements and compounds in aqueous environments, including natural waters, sediments and soils. It is well suited to in situ detection of bioavailable toxic trace metal contaminants. The technique involves using a specially-designed passive sampler that houses a binding gel, diffusive gel and membrane filter. The element or compound passes through the membrane filter and diffusive gel and is assimilated by the binding gel in a rate-controlled manner. Post-deployment analysis of the binding gel can be used to determine the time-weighted-average bulk solution concentration of the element or compound via a simple equation.
Bioavailability, in environmental and soil sciences, represents the amount of an element or compound that is accessible to an organism for uptake or adsorption across its cellular membrane. In environmental and agricultural applications, bioavailability most often refers to availability of contaminants, such as organic pollutants or heavy metals, in soil systems and is also used frequently in determining potential risk of land application of sewage sludge or other inorganic/organic waste materials.
Semipermeable membrane devices (SPMD) are passive sampling devices used to monitor trace levels of organic compounds with a log Kow > 3. SPMDs are an effective way of monitoring the concentrations of chemicals from anthropogenic runoff and pollution in the marine environment because of their ability to detect minuscule levels of chemical. The data collected from a passive sampler is important for examining the amount of chemical in the environment and can therefore be used to formulate other scientific research about the effects of those chemicals on the organisms as well as the environment. Examples of chemicals commonly measured using SPMDs include: PAHs, PCBs, PBDEs, dioxins and furans as well as hydrophobic waste-water effluents like fragrances, triclosan, and phthalates.
The stabilized liquid membrane device (SLMD) is a passive, integrative sampler that provides an alternative or complementary approach to the conventional water sampling of aqueous metals. The simple device is composed of nonporous low-density plastic lay-flat tubing, which is filled with a chemical mixture containing a chelating agent (metal-binding agent) and a long chain organic acid. The water-insoluble chelating agent-organic acid mixture diffuses in a controlled manner to the exterior surface of the sampler membrane and binds to environmental metals. In practice, the SLMD provides for continuous sequestration of bioavailable forms of trace metals, such as, cadmium (Cd), cobalt (Co), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). The SLMD can also be utilized for in-laboratory preconcentration and speciation of bioavailable trace metals from grab water samples.
In 2015, 251 million tubes of toothpaste were sold in the United States. A single tube holds roughly 170 grams of toothpaste, so approximately 43 kilotonnes of toothpaste get washed into the water systems annually. Toothpaste contains silver nanoparticles, also known as nanosilver or AgNPs, among other compounds.

Passive sampling is an environmental monitoring technique involving the use of a collecting medium, such as a man-made device or biological organism, to accumulate chemical pollutants in the environment over time. This is in contrast to grab sampling, which involves taking a sample directly from the media of interest at one point in time. In passive sampling, average chemical concentrations are calculated over a device's deployment time, which avoids the need to visit a sampling site multiple times to collect multiple representative samples. Currently, passive samplers have been developed and deployed to detect toxic metals, pesticides, pharmaceuticals, radionuclides, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and other organic compounds in water, while some passive samplers can detect hazardous substances in the air.








