
Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to reduce symptoms. Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.
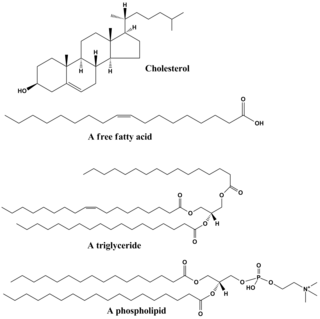
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
The historical application of biotechnology throughout time is provided below in chronological order.

Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, and in both fatty acid and amino acid metabolism. It is important in the normal functioning of the nervous system via its role in the synthesis of myelin, and in the circulatory system in the maturation of red blood cells in the bone marrow. Plants do not need cobalamin and carry out the reactions with enzymes that are not dependent on it.

Fluorine is a chemical element; it has symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is extremely reactive, as it reacts with all other elements except for the light inert gases.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.
Streptomyces cinnamoneus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Japan. Streptomyces cinnamoneus produces duramycin A, duramycin B, duramycin C, carbomycin, cinnomycin and fungichromin.
Streptomyces galbus is a bacterium species from the genus of Streptomyces which has been isolated from soil from West Bengal. Streptomyces galbus produces xylanase, galbonolides A, galbonolides B and the actinomycin X complex.
Streptomyces niveus is a bacterium species from the genus of Streptomyces which has been isolated from soil in the United States. Streptomyces niveus produces the aminocoumarin antibiotic novobiocin and the compounds nivetetracyclate A and nivetetracyclate B.
Streptomyces phaeochromogenes is a bacterium species from the genus of Streptomyces. Streptomyces phaeochromogenes produces tyrosinate, bromoperoxidase, ditryptophenalin, phaeochromycin A, phaeochromycin B, phaeochromycin C, phaeochromycin D and phaeochromycin E. Streptomyces phaeochromogenes also produces moenomycin and bambermycin.
Tsukamurella pulmonis is a Gram-positive and aerobic bacterium from the genus Tsukamurella which has been isolated from the sputum from a patient with lung tuberculosis in Germany.
Lydicamycin is an organic compound with the molecular formula C47H74N4O10. Lydicamycin is an antibiotic with activity against Gram-positive bacteria. The bacteria Streptomyces lydicamycinicus and Streptomyces platensis produces lydicamycin.
This article lists a number of significant events in science that have occurred in the first quarter of 2023.






