
Aspergillus niger is a mold classified within the Nigri section of the Aspergillus genus. The Aspergillus genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on decomposing matter, and suspended in the air. Species within this genus often grow quickly and can sporulate within a few days of germination. A combination of characteristics unique to A. niger makes the microbe invaluable to the production of many acids, proteins and bioactive compounds. Characteristics including extensive metabolic diversity, high production yield, secretion capability, and the ability to conduct post-translational modifications are responsible for A. niger's robust production of secondary metabolites. A. niger's capability to withstand extremely acidic conditions makes it especially important to the industrial production of citric acid.

Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:

An exoenzyme, or extracellular enzyme, is an enzyme that is secreted by a cell and functions outside that cell. Exoenzymes are produced by both prokaryotic and eukaryotic cells and have been shown to be a crucial component of many biological processes. Most often these enzymes are involved in the breakdown of larger macromolecules. The breakdown of these larger macromolecules is critical for allowing their constituents to pass through the cell membrane and enter into the cell. For humans and other complex organisms, this process is best characterized by the digestive system which breaks down solid food via exoenzymes. The small molecules, generated by the exoenzyme activity, enter into cells and are utilized for various cellular functions. Bacteria and fungi also produce exoenzymes to digest nutrients in their environment, and these organisms can be used to conduct laboratory assays to identify the presence and function of such exoenzymes. Some pathogenic species also use exoenzymes as virulence factors to assist in the spread of these disease-causing microorganisms. In addition to the integral roles in biological systems, different classes of microbial exoenzymes have been used by humans since pre-historic times for such diverse purposes as food production, biofuels, textile production and in the paper industry. Another important role that microbial exoenzymes serve is in the natural ecology and bioremediation of terrestrial and marine environments.

Phycocyanin is a pigment-protein complex from the light-harvesting phycobiliprotein family, along with allophycocyanin and phycoerythrin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble, so they cannot exist within the membrane like carotenoids can. Instead, phycobiliproteins aggregate to form clusters that adhere to the membrane called phycobilisomes. Phycocyanin is a characteristic light blue color, absorbing orange and red light, particularly near 620 nm, and emits fluorescence at about 650 nm. Allophycocyanin absorbs and emits at longer wavelengths than phycocyanin C or phycocyanin R. Phycocyanins are found in cyanobacteria. Phycobiliproteins have fluorescent properties that are used in immunoassay kits. Phycocyanin is from the Greek phyco meaning “algae” and cyanin is from the English word “cyan", which conventionally means a shade of blue-green and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue". The product phycocyanin, produced by Aphanizomenon flos-aquae and Spirulina, is for example used in the food and beverage industry as the natural coloring agent 'Lina Blue' or 'EXBERRY Shade Blue' and is found in sweets and ice cream. In addition, fluorescence detection of phycocyanin pigments in water samples is a useful method to monitor cyanobacteria biomass.
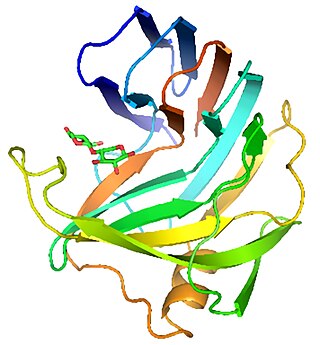
Endo-1,4-β-xylanase is any of a class of enzymes that degrade the linear polysaccharide xylan into xylose, thus breaking down hemicellulose, one of the major components of plant cell walls:

Xylan is a type of hemicellulose, a polysaccharide consisting mainly of xylose residues. It is found in plants, in the secondary cell walls of dicots and all cell walls of grasses. Xylan is the third most abundant biopolymer on Earth, after cellulose and chitin.

Lymphocyte cytosolic protein 2, also known as LCP2 or SLP-76, is a signal-transducing adaptor protein expressed in T cells and myeloid cells and is important in the signaling of T-cell receptors (TCRs). As an adaptor protein, SLP-76 does not have catalytic functions, primarily binding other signaling proteins to form larger signaling complexes. It is a key component of the signaling pathways of receptors with immunoreceptor tyrosine-based activation motifs (ITAMs) such as T-cell receptors, its precursors, and receptors for the Fc regions of certain antibodies. SLP-76 is expressed in T-cells and related lymphocytes like natural killer cells.
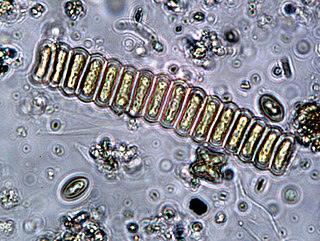
Scenedesmus is a genus of green algae, in the class Chlorophyceae. They are colonial and non-motile. They are one of the most common components of phytoplankton in freshwater habitats worldwide.

The muscarinic acetylcholine receptor M4, also known as the cholinergic receptor, muscarinic 4 (CHRM4), is a protein that, in humans, is encoded by the CHRM4 gene.

Retinal pigment epithelium-specific 65 kDa protein, also known as retinoid isomerohydrolase, is an enzyme of the vertebrate visual cycle that is encoded in humans by the RPE65 gene. RPE65 is expressed in the retinal pigment epithelium and is responsible for the conversion of all-trans-retinyl esters to 11-cis-retinol during phototransduction. 11-cis-retinol is then used in visual pigment regeneration in photoreceptor cells. RPE65 belongs to the carotenoid oxygenase family of enzymes.

Nuclear distribution protein nudE-like 1 is a protein that in humans is encoded by the NDEL1 gene.

Neuroblast differentiation-associated protein AHNAK, also known as desmoyokin, is a protein that in humans is encoded by the AHNAK gene. AHNAK was originally identified in 1989 and named desmoyokin due to its localization pattern in the desmosomal plaque. AHNAK has been shown to be essential for pseudopod protrusion and cell migration.

Neurolysin, mitochondrial is a protein that in humans is encoded by the NLN gene. It is a 78-kDa enzyme, widely distributed in mammalian tissues and found in various subcellular locations that vary with cell type. Neurolysin exemplifies the ability of neuropeptidases to target various cleavage site sequences by hydrolyzing them in vitro, and metabolism of neurotensin is the most important role of neurolysin in vivo. Neurolysin has also been implicated in pain control, blood pressure regulation, sepsis, reproduction, cancer biology pathogenesis of stroke, and glucose metabolism.

Carbohydrate sulfotransferase 11 is an enzyme that in humans is encoded by the CHST11 gene.

Interferon alpha-16, also known as IFN-alpha-16, is a protein that in humans is encoded by theIFNA16 gene.

Glucanases are enzymes that break down large polysaccharides via hydrolysis. The product of the hydrolysis reaction is called a glucan, a linear polysaccharide made of up to 1200 glucose monomers, held together with glycosidic bonds. Glucans are abundant in the endosperm cell walls of cereals such as barley, rye, sorghum, rice, and wheat. Glucanases are also referred to as lichenases, hydrolases, glycosidases, glycosyl hydrolases, and/or laminarinases. Many types of glucanases share similar amino acid sequences but vastly different substrates. Of the known endo-glucanases, 1,3-1,4-β-glucanase is considered the most active.

Extracellular enzymes or exoenzymes are synthesized inside the cell and then secreted outside the cell, where their function is to break down complex macromolecules into smaller units to be taken up by the cell for growth and assimilation. These enzymes degrade complex organic matter such as cellulose and hemicellulose into simple sugars that enzyme-producing organisms use as a source of carbon, energy, and nutrients. Grouped as hydrolases, lyases, oxidoreductases and transferases, these extracellular enzymes control soil enzyme activity through efficient degradation of biopolymers.
Streptomyces malaysiensis is a streptomycete bacterium species. At maturity, the aerial hyphae of this species differentiates into tight, spiral chains of rugose, cylindrical spores. Its type strain is ATB-11T.
Streptomyces drozdowiczii is a cellulolytic bacterium species from the genus of Streptomyces which has been isolated from soil from the Mata Atlântica forest in Brazil.
Streptomyces misionensis is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces misionensis produces misionin.















