
A hydrogel is a biphasic material, a mixture of porous, permeable solids and at least 10% by weight or volume of interstitial fluid composed completely or mainly by water. In hydrogels the porous permeable solid is a water insoluble three dimensional network of natural or synthetic polymers and a fluid, having absorbed a large amount of water or biological fluids. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature. The term 'hydrogel' was coined in 1894.
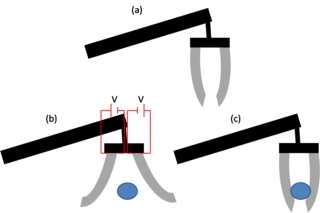
An electroactive polymer (EAP) is a polymer that exhibits a change in size or shape when stimulated by an electric field. The most common applications of this type of material are in actuators and sensors. A typical characteristic property of an EAP is that they will undergo a large amount of deformation while sustaining large forces.
Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult, and the reduced ability to adjust the dosages.
Modified-release dosage is a mechanism that delivers a drug with a delay after its administration or for a prolonged period of time or to a specific target in the body.
Poly(N-isopropylacrylamide) (variously abbreviated PNIPA, PNIPAM, PNIPAAm, NIPA, PNIPAA or PNIPAm) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
Smart polymers, stimuli-responsive polymers or functional polymers are high-performance polymers that change according to the environment they are in.
Nanocomposite hydrogels are nanomaterial-filled, hydrated, polymeric networks that exhibit higher elasticity and strength relative to traditionally made hydrogels. A range of natural and synthetic polymers are used to design nanocomposite network. By controlling the interactions between nanoparticles and polymer chains, a range of physical, chemical, and biological properties can be engineered. The combination of organic (polymer) and inorganic (clay) structure gives these hydrogels improved physical, chemical, electrical, biological, and swelling/de-swelling properties that cannot be achieved by either material alone. Inspired by flexible biological tissues, researchers incorporate carbon-based, polymeric, ceramic and/or metallic nanomaterials to give these hydrogels superior characteristics like optical properties and stimulus-sensitivity which can potentially be very helpful to medical and mechanical fields.
Hydrogels are three-dimensional networks consisting of chemically or physically cross-linked hydrophilic polymers. The insoluble hydrophilic structures absorb polar wound exudates and allow oxygen diffusion at the wound bed to accelerate healing. Hydrogel dressings can be designed to prevent bacterial infection, retain moisture, promote optimum adhesion to tissues, and satisfy the basic requirements of biocompatibility. Hydrogel dressings can also be designed to respond to changes in the microenvironment at the wound bed. Hydrogel dressings should promote an appropriate microenvironment for angiogenesis, recruitment of fibroblasts, and cellular proliferation.

Ophthalmic drug administration is the administration of a drug to the eyes, most typically as an eye drop formulation. Topical formulations are used to combat a multitude of diseased states of the eye. These states may include bacterial infections, eye injury, glaucoma, and dry eye. However, there are many challenges associated with topical delivery of drugs to the cornea of the eye.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.

Dextran drug delivery systems involve the use of the natural glucose polymer dextran in applications as a prodrug, nanoparticle, microsphere, micelle, and hydrogel drug carrier in the field of targeted and controlled drug delivery. According to several in vitro and animal research studies, dextran carriers reduce off-site toxicity and improve local drug concentration at the target tissue site. This technology has significant implications as a potential strategy for delivering therapeutics to treat cancer, cardiovascular diseases, pulmonary diseases, bone diseases, liver diseases, colonic diseases, infections, and HIV.
Conventional drug delivery is limited by the inability to control dosing, target specific sites, and achieve targeted permeability. Traditional methods of delivering therapeutics to the body experience challenges in achieving and maintaining maximum therapeutic effect while avoiding the effects of drug toxicity. Many drugs that are delivered orally or parenterally do not include mechanisms for sustained release, and as a result they require higher and more frequent dosing to achieve any therapeutic effect for the patient. As a result, the field of drug delivery systems developed into a large focus area for pharmaceutical research to address these limitations and improve quality of care for patients. Within the broad field of drug delivery, the development of stimuli-responsive drug delivery systems has created the ability to tune drug delivery systems to achieve more controlled dosing and targeted specificity based on material response to exogenous and endogenous stimuli.
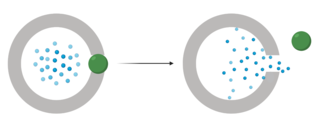
Gated drug delivery systems are a method of controlled drug release that center around the use of physical molecules that cover the pores of drug carriers until triggered for removal by an external stimulus. Gated drug delivery systems are a recent innovation in the field of drug delivery and pose as a promising candidate for future drug delivery systems that are effective at targeting certain sites without having leakages or off target effects in normal tissues. This new technology has the potential to be used in a variety of tissues over a wide range of disease states and has the added benefit of protecting healthy tissues and reducing systemic side effects.
Pullulan bioconjugates are systems that use pullulan as a scaffold to attach biological materials to, such as drugs. These systems can be used to enhance the delivery of drugs to specific environments or the mechanism of delivery. These systems can be used in order to deliver drugs in response to stimuli, create a more controlled and sustained release, and provide a more targeted delivery of certain drugs.
Reduction-sensitive nanoparticles (RSNP) consist of nanocarriers that are chemically responsive to reduction. Drug delivery systems using RSNP can be loaded with different drugs that are designed to be released within a concentrated reducing environment, such as the tumor-targeted microenvironment. Reduction-Sensitive Nanoparticles provide an efficient method of targeted drug delivery for the improved controlled release of medication within localized areas of the body.
pH-responsive tumor-targeted drug delivery is a specialized form of targeted drug delivery that utilizes nanoparticles to deliver therapeutic drugs directly to cancerous tumor tissue while minimizing its interaction with healthy tissue. Scientists have used drug delivery as a way to modify the pharmacokinetics and targeted action of a drug by combining it with various excipients, drug carriers, and medical devices. These drug delivery systems have been created to react to the pH environment of diseased or cancerous tissues, triggering structural and chemical changes within the drug delivery system. This form of targeted drug delivery is to localize drug delivery, prolongs the drug's effect, and protect the drug from being broken down or eliminated by the body before it reaches the tumor.
Bioprinting drug delivery is a method of using the three-dimensional printing of biomaterials through an additive manufacturing technique to develop drug delivery vehicles that are biocompatible tissue-specific hydrogels or implantable devices. 3D bioprinting uses printed cells and biological molecules to manufacture tissues, organs, or biological materials in a scaffold-free manner that mimics living human tissue to provide localized and tissue-specific drug delivery, allowing for targeted disease treatments with scalable and complex geometry.
Ultrasound-triggered drug delivery using stimuli-responsive hydrogels refers to the process of using ultrasound energy for inducing drug release from hydrogels that are sensitive to acoustic stimuli. This method of approach is one of many stimuli-responsive drug delivery-based systems that has gained traction in recent years due to its demonstration of localization and specificity of disease treatment. Although recent developments in this field highlight its potential in treating certain diseases such as COVID-19, there remain many major challenges that need to be addressed and overcome before more related biomedical applications are clinically translated into standard of care.
Intranasal drug delivery occurs when particles are inhaled into the nasal cavity and transported directly into the nervous system. Though pharmaceuticals can be injected into the nose, some concerns include injuries, infection, and safe disposal. Studies demonstrate improved patient compliance with inhalation. Treating brain diseases has been a challenge due to the blood brain barrier. Previous studies evaluated the efficacy of delivery therapeutics through intranasal route for brain diseases and mental health conditions. Intranasal administration is a potential route associated with high drug transfer from nose to brain and drug bioavailability.








