
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.
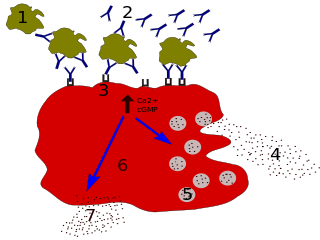
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.
Interleukins (ILs) are a group of cytokines that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related proteins.

Interleukin 6 (IL-6) is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. In humans, it is encoded by the IL6 gene.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.

Charles A. Dinarello is a professor of medicine at the University of Colorado at Denver. He is an expert on inflammatory cytokines, specifically Interleukin 1.
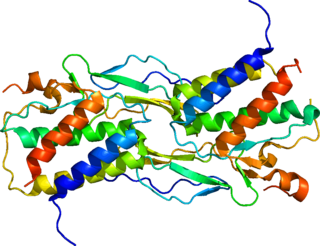
Interleukin-15 (IL-15) is a protein that in humans is encoded by the IL15 gene. IL-15 is an inflammatory cytokine with structural similarity to Interleukin-2 (IL-2). Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain. IL-15 is secreted by mononuclear phagocytes following infection by virus(es). This cytokine induces the proliferation of natural killer cells, i.e. cells of the innate immune system whose principal role is to kill virally infected cells.

Interleukin 21 (IL-21) is a protein that in humans is encoded by the IL21 gene.

Interleukin 20 (IL20) is a protein that is in humans encoded by the IL20 gene which is located in close proximity to the IL-10 gene on the 1q32 chromosome. IL-20 is a part of an IL-20 subfamily which is a part of a larger IL-10 family.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

Glycoprotein 130 is a transmembrane protein which is the founding member of the class of tall cytokine receptors. It forms one subunit of the type I cytokine receptor within the IL-6 receptor family. It is often referred to as the common gp130 subunit, and is important for signal transduction following cytokine engagement. As with other type I cytokine receptors, gp130 possesses a WSXWS amino acid motif that ensures correct protein folding and ligand binding. It interacts with Janus kinases to elicit an intracellular signal following receptor interaction with its ligand. Structurally, gp130 is composed of five fibronectin type-III domains and one immunoglobulin-like C2-type (immunoglobulin-like) domain in its extracellular portion.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, a severe form of arthritis in children, and COVID‑19. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.

Interleukin 6 receptor (IL6R) also known as CD126 is a type I cytokine receptor.

Interleukin-17A is a protein that in humans is encoded by the IL17A gene. In rodents, IL-17A used to be referred to as CTLA8, after the similarity with a viral gene.
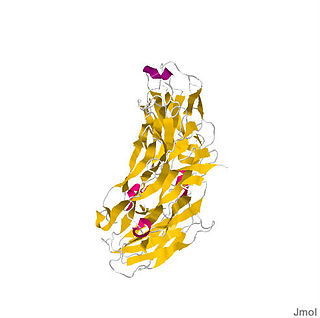
Interleukin-17 receptor (IL-17R) is a cytokine receptor which belongs to new subfamily of receptors binding proinflammatory cytokine interleukin 17A, a member of IL-17 family ligands produced by T helper 17 cells (Th17). IL-17R family consists of 5 members: IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE. Functional IL-17R is a transmembrane receptor complex usually consisting of one IL-17RA, which is a founding member of the family, and second other family subunit, thus forming heteromeric receptor binding different ligands. IL-17A, a founding member of IL-17 ligand family binds to heteromeric IL-17RA/RC receptor complex. IL-17RB binds preferentially IL-17B and IL-17E and heteromeric IL-17RA/RE complex binds IL-17C. However, there is still unknown ligand for IL-17RD. The first identified member IL-17RA is located on human chromosome 22, whereas other subunits IL-17RB to IL-17RD are encoded within human chromosome 3.

The Interleukin-1 family is a group of 11 cytokines that plays a central role in the regulation of immune and inflammatory responses to infections or sterile insults.

Interleukin 23 (IL-23) is a heterodimeric cytokine composed of an IL-12B (IL-12p40) subunit and an IL-23A (IL-23p19) subunit. IL-23 is part of the IL-12 family of cytokines. The functional receptor for IL-23 consists of a heterodimer between IL-12Rβ1 and IL-23R.

Tasuku Honjo is a Japanese physician-scientist and immunologist. He won the 2018 Nobel Prize in Physiology or Medicine and is best known for his identification of programmed cell death protein 1 (PD-1). He is also known for his molecular identification of cytokines: IL-4 and IL-5, as well as the discovery of activation-induced cytidine deaminase (AID) that is essential for class switch recombination and somatic hypermutation.

Olamkicept, also known as soluble gp130Fc or sgp130Fc is an immunosuppressive drug candidate, which selectively blocks activities of the cytokine Interleukin-6, which are mediated by the soluble Interleukin-6. Interleukin-6 is a cytokine, which plays a dominant role in the regulation of the immune response and also in autoimmunity. Furthermore, Interleukin-6 has been demonstrated to be involved in the regulation of metabolism and body weight. Interleukin-6 also has many activities on neural cells. The biochemical principle was invented by the German biochemist Stefan Rose-John and it was further developed into a biotech compound by the Conaris Research Institute AG, which gave an exclusive world-wide license to the Swiss-based biopharmaceutical company Ferring Pharmaceuticals. In December 2016, Ferring and the biotech company I-MAB signed a licensing agreement granting I-MAB exclusive rights in Asia to Olamkicept for the treatment of autoimmune disease.
Hyper-IL-6 is a designer cytokine, which was generated by the German biochemist Stefan Rose-John. Hyper-IL-6 is a fusion protein of the four-helical cytokine Interleukin-6 and the soluble Interleukin-6 receptor which are covalently linked by a flexible peptide linker. Interleukin-6 on target cells binds to a membrane bound Interleukin-6 receptor. The complex of Interleukin-6 and the Interleukin-6 receptor associate with a second receptor protein called gp130, which dimerises and initiates intracellular signal transduction. Gp130 is expressed on all cells of the human body whereas the Interleukin-6 receptor is only found on few cells such as hepatocytes and some leukocytes. Neither Interleukin-6 nor the Interleukin-6 receptor have a measurable affinity for gp130. Therefore, cells, which only express gp130 but no Interleukin-6 receptor are not responsive to Interleukin-6. It was found, however, that the membrane-bound Interleukin-6 receptor can be cleaved from the cell membrane generating a soluble Interleukin-6 receptor. The soluble Interleukin-6 receptor can bind the ligand Interleukin-6 with similar affinity as the membrane-bound Interleukin-6 receptor and the complex of Interleukin-6 and the soluble Interleukin-6 receptor can bind to gp130 on cells, which only express gp130 but no Interleukin-6 receptor. The mode of signaling via the soluble Interleukin-6 receptor has been named Interleukin-6 trans-signaling whereas Interleukin-6 signaling via the membrane-bound Interleukin-6 receptor is referred to as Interleukin-6 classic signaling. Therefore, the generation of the soluble Interleukin-6 receptor enables cells to respond to Interleukin-6, which in the absence of soluble Interleukin-6 receptor would be completely unresponsive to the cytokine.


















