
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Methyl tert-butyl ether (MTBE), also known as tert-butyl methyl ether, is an organic compound with a structural formula (CH3)3COCH3. MTBE is a volatile, flammable, and colorless liquid that is sparingly soluble in water. Primarily used as a fuel additive, MTBE is blended into gasoline to increase its octane rating and knock resistance, and reduce unwanted emissions.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

Ethyl tertiary-butyl ether (ETBE), also known as ethyl tert-butyl ether, is commonly used as an oxygenate gasoline additive in the production of gasoline from crude oil. ETBE offers equal or greater air quality benefits than ethanol, while being technically and logistically less challenging. Unlike ethanol, ETBE does not induce evaporation of gasoline, which is one of the causes of smog, and does not absorb moisture from the atmosphere.

Di-tert-butyl ether is a tertiary ether, primarily of theoretical interest as the simplest member of the class of di-tertiary ethers.
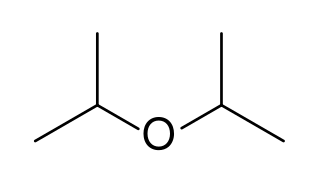
Diisopropyl ether is a secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with organic solvents. It is used as an extractant and an oxygenate gasoline additive. It is obtained industrially as a byproduct in the production of isopropanol by hydration of propylene. Diisopropyl ether is sometimes represented by the abbreviation DIPE.
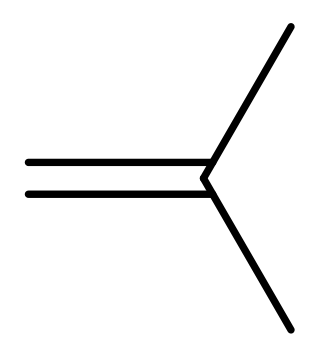
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.

Butan-2-ol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol, isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-butan-2-ol and (S)-(+)-butan-2-ol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
Oxygenated chemical compounds are hydrocarbons which contain at least one oxygen atom as a part of their chemical structure. The term often refers to oxygenated chemical compounds added to fuels. Oxygenates are usually employed as gasoline additives to reduce carbon monoxide and soot that is created during the burning of the fuel. Compounds related to soot, such as polyaromatic hydrocarbons (PAHs) and nitrated PAHs, are also reduced.
The molecular formula C4H10O may refer to:
The molecular formula C5H10O2 (molar mass: 102.13 g/mol) may refer to:
The molecular formula C5H12O (molar mass: 88.15 g/mol, exact mass: 88.088815) may refer to:
The molecular formula C6H14O may refer to:
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha. It has an ethereous odor. Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.

tert-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent.
Methylibium petroleiphilum is a species of methyl tert-butyl ether-degrading methylotroph, the type species of its genus. It is a Gram-negative, rod-shaped, motile, non-pigmented, facultative aerobe, with type strain PM1T.
Ether cleavage refers to chemical substitution reactions that lead to the cleavage of ethers. Due to the high chemical stability of ethers, the cleavage of the C-O bond is uncommon in the absence of specialized reagents or under extreme conditions.












