
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, sweet-tasting, viscous liquid.

Thermal radiation is electromagnetic radiation generated by the thermal motion of particles in matter. Thermal radiation is generated when heat from the movement of charges in the material is converted to electromagnetic radiation. All matter with a temperature greater than absolute zero emits thermal radiation. Particle motion results in charge-acceleration or dipole oscillation which produces electromagnetic radiation.
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. It is a grey solid. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.
Brake fluid is a type of hydraulic fluid used in hydraulic brake and hydraulic clutch applications in automobiles, motorcycles, light trucks, and some bicycles. It is used to transfer force into pressure, and to amplify braking force. It works because liquids are not appreciably compressible.
A coolant is a substance, typically liquid or gas, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.
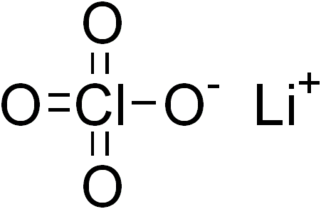
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.

Paraformaldehyde (PFA) is the smallest polyoxymethylene, the polymerization product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition. Paraformaldehyde is a poly-acetal.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Diglyme, or bis(2-methoxyethyl) ether, is a solvent with a high boiling point. It is an organic compound which is the dimethyl ether of diethylene glycol. It is a colorless liquid with a slight ether-like odor. It is miscible with water as well as organic solvents.
Solar air conditioning refers to any air conditioning (cooling) system that uses solar power.

Syringol is a naturally occurring aromatic organic compound. It is a dimethyl ether of pyrogallol.
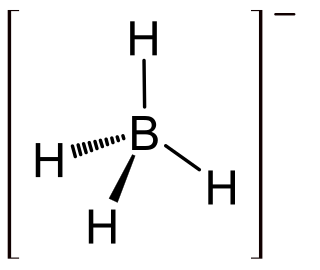
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.

Dansyl chloride or 5-(DimethylAmino)Naphthalene-1-SulfonYL chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. It can also be made to react with secondary amines. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing and amino acid analysis. Dansyl chloride may also be denoted DNSC. Likewise, a similar derivative, dansyl amide is known as DNSA.

Bakthan Singaram is a professor of organic chemistry at the University of California, Santa Cruz in Santa Cruz, California, where he has taught since 1989. Singaram's primary focus is in the area of boron-based organic chemistry. He gained his Ph.D. from the University of Madras, Tamil Nadu, India in 1977. Singaram also worked in and directed the laboratory of Nobel Prize-winning chemist Herbert Brown, who shared the 1979 Nobel prize in chemistry 1979 with Georg Wittig "for their development of the use of boron- and phosphorus-containing compounds, respectively, into important reagents in organic synthesis". Singaram then left the Brown research group to take a position as an assistant professor in 1989 at the University of California, Santa Cruz, where he remains today. Singaram has also acted as a visiting professor at several universities, such as the University of Puerto Rico and the University of Rennes 1 in Rennes, France. Most recently, he received an award from The Boron in the Americas (BORAM) Organization presented at the Regular BORAM Awards in June 2012.

Lithium cobalt oxide, sometimes called lithium cobaltate or lithium cobaltite, is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.

Methional is an organic compound with the formula CH3SCH2CH2CHO. It is a colorless liquid that is a degradation product of methionine. It is a notable flavor in potato-based snacks, namely potato chips, one of the most popular foods containing methional. Traces of the compound can also be found in black tea and green tea based products. Methional contains both aldehyde and thioether functional groups. It is readily soluble in alcohol solvents, including propylene glycol and dipropylene glycol.

Propylene glycol methyl ether acetate is a P-type glycol ether used in inks, coatings, and cleaners. It is sold by Dow Chemical under the name Dowanol PMA, by Shell Chemical under the name methyl proxitol acetate, and by Eastman under the name PM Acetate.
Polytetrafluoroethylene (PTFE), better known by its trade name Teflon, has many desirable properties which make it an attractive material for numerous industries. It has good chemical resistance, a low dielectric constant, low dielectric loss, and a low coefficient of friction, making it ideal for reactor linings, circuit boards, and kitchen utensils, to name a few applications. However, its nonstick properties make it challenging to bond to other materials or to itself.

A solid-state electrolyte (SSE) is a solid ionic conductor and electron-insulating material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1 and a reduction potential of -3.04 V vs SHE, in substitution of the traditional low capacity graphite, which exhibits a theoretical capacity of 372 mAh g−1 in its fully lithiated state of LiC6, is the first step in the realization of a lighter, thinner and cheaper rechargeable battery. Moreover this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle. Despite the promising advantages, there are still many limitations that are hindering the transition of SSEs from academia research to large-scale production, depending mainly on the poor ionic conductivity compared to that of liquid counterparts. However, many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.
 [3]
[3] 













