
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

Selected-ion flow-tube mass spectrometry (SIFT-MS) is a quantitative mass spectrometry technique for trace gas analysis which involves the chemical ionization of trace volatile compounds by selected positive precursor ions during a well-defined time period along a flow tube. Absolute concentrations of trace compounds present in air, breath or the headspace of bottled liquid samples can be calculated in real time from the ratio of the precursor and product ion signal ratios, without the need for sample preparation or calibration with standard mixtures. The detection limit of commercially available SIFT-MS instruments extends to the single digit pptv range.

Prismane or 'Ladenburg benzene' is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are arranged in the shape of a six-atom triangular prism—this compound is the parent and simplest member of the prismanes class of molecules. Albert Ladenburg proposed this structure for the compound now known as benzene. The compound was not synthesized until 1973.

Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPLC). APCI is a soft ionization method similar to chemical ionization where primary ions are produced on a solvent spray. The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da. The application of APCI with HPLC has gained a large popularity in trace analysis detection such as steroids, pesticides and also in pharmacology for drug metabolites.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.
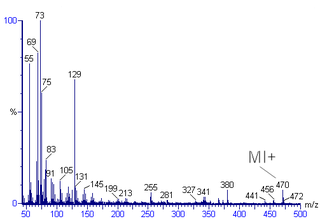
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It is commonly used for the identification of organic compounds from electron ionization mass spectrometry. Organic chemists obtain mass spectra of chemical compounds as part of structure elucidation and the analysis is part of many organic chemistry curricula.
Electron capture ionization is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form . The reaction is

In mass spectrometry, fragmentation is the dissociation of energetically unstable molecular ions formed from passing the molecules mass spectrum. These reactions are well documented over the decades and fragmentation patterns are useful to determine the molar weight and structural information of unknown molecules. Fragmentation that occurs in tandem mass spectrometry experiments has been a recent focus of research, because this data helps facilitate the identification of molecules.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.

Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity.

Octaazacubane is a hypothetical explosive allotrope of nitrogen with formula N8, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a cubane-type cluster, where all eight corners are nitrogen atoms bonded along the edges. It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N–N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry.
Nitrogen pentafluoride is a theoretical compound of nitrogen and fluorine with the chemical formula NF5. It is hypothesized to exist based on the existence of the pentafluorides of the atoms below nitrogen in the periodic table, such as phosphorus pentafluoride. Theoretical models of the nitrogen pentafluoride molecule are either a trigonal bipyramidal covalently bound molecule with symmetry group D3h, or [NF4]+F−, which would be an ionic solid.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.

Phosphorus mononitride is an inorganic compound with the chemical formula PN. Containing only phosphorus and nitrogen, this material is classified as a binary nitride. From the Lewis structure perspective, it can be represented with a P-N triple bond with a lone pair on each atom. It is isoelectronic with N2, CO, P2, CS and SiO.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.

Superelectrophilic anions are a class of molecular ions that exhibit highly electrophilic reaction behavior despite their overall negative charge. Thus, they are even able to bind the unreactive noble gases or molecular nitrogen at room temperature. The only representatives known so far are the fragment ions of the type [B12X11]– derived from the closo-dodecaborate dianions [B12X12]2–. X represents a substituent connected to a boron atom (cf. Fig. 1). For this reason, the following article deals exclusively with superelectrophilic anions of this type.
Pnictogen-substituted tetrahedranes are pnictogen-containing analogues of tetrahedranes with the formula RxCxPn4-x. Computational work has indicated that the incorporation of pnictogens to the tetrahedral core alleviates the ring strain of tetrahedrane. Although theoretical work on pnictogen-substituted tetrahedranes has existed for decades, only the phosphorus-containing species have been synthesized. These species exhibit novel reactivities, most often through ring-opening and polymerization pathways. Phosphatetrahedranes are of interest as new retrons for organophosphorus chemistry. Their strain also make them of interest in the development of energy-dense compounds.











