
The Apicomplexa are organisms of a large phylum of mainly parasitic alveolates. Most possess a unique form of organelle structure that comprises a type of non-photosynthetic plastid called an apicoplast—with an apical complex membrane. The organelle's apical shape is an adaptation that the apicomplexan applies in penetrating a host cell.

Babesiosis or piroplasmosis is a malaria-like parasitic disease caused by infection with a eukaryotic parasite in the order Piroplasmida, typically a Babesia or Theileria, in the phylum Apicomplexa. Human babesiosis transmission via tick bite is most common in the Northeastern and Midwestern United States and parts of Europe, and sporadic throughout the rest of the world. It occurs in warm weather. People can get infected with Babesia parasites by the bite of an infected tick, by getting a blood transfusion from an infected donor of blood products, or by congenital transmission . Ticks transmit the human strain of babesiosis, so it often presents with other tick-borne illnesses such as Lyme disease. After trypanosomes, Babesia is thought to be the second-most common blood parasite of mammals. They can have major adverse effects on the health of domestic animals in areas without severe winters. In cattle, the disease is known as Texas cattle fever or redwater.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.

Plasmodium malariae is a parasitic protozoan that causes malaria in humans. It is one of several species of Plasmodium parasites that infect other organisms as pathogens, also including Plasmodium falciparum and Plasmodium vivax, responsible for most malarial infection. Found worldwide, it causes a so-called "benign malaria", not nearly as dangerous as that produced by P. falciparum or P. vivax. The signs include fevers that recur at approximately three-day intervals – a quartan fever or quartan malaria – longer than the two-day (tertian) intervals of the other malarial parasite.

Eimeria is a genus of apicomplexan parasites that includes various species capable of causing the disease coccidiosis in animals such as cattle, poultry and smaller ruminants including sheep and goats. Eimeria species are considered to be monoxenous because the life cycle is completed within a single host, and stenoxenous because they tend to be host specific, although a number of exceptions have been identified. Species of this genus infect a wide variety of hosts. Thirty-one species are known to occur in bats (Chiroptera), two in turtles, and 130 named species infect fish. Two species infect seals. Five species infect llamas and alpacas: E. alpacae, E. ivitaensis, E. lamae, E. macusaniensis, and E. punonensis. A number of species infect rodents, including E. couesii, E. kinsellai, E. palustris, E. ojastii and E. oryzomysi. Others infect poultry, rabbits and cattle. For full species list, see below.

Plasmodium knowlesi is a parasite that causes malaria in humans and other primates. It is found throughout Southeast Asia, and is the most common cause of human malaria in Malaysia. Like other Plasmodium species, P. knowlesi has a life cycle that requires infection of both a mosquito and a warm-blooded host. While the natural warm-blooded hosts of P. knowlesi are likely various Old World monkeys, humans can be infected by P. knowlesi if they are fed upon by infected mosquitoes. P. knowlesi is a eukaryote in the phylum Apicomplexa, genus Plasmodium, and subgenus Plasmodium. It is most closely related to the human parasite Plasmodium vivax as well as other Plasmodium species that infect non-human primates.
Cytauxzoon felis is a protozoal organism transmitted to domestic cats by tick bites, and whose natural reservoir host is the bobcat. C. felis has been found in other wild felid species such as the cougar, as well as a white tiger in captivity. C. felis infection is limited to the family Felidae which means that C. felis poses no zoonotic risk or agricultural risk. Until recently it was believed that after infection with C. felis, pet cats almost always died. As awareness of C. felis has increased it has been found that treatment is not always futile. More cats have been shown to survive the infection than was previously thought. New treatments offer as much as 60% survival rate.

Babesia, also called Nuttallia, is an apicomplexan parasite that infects red blood cells and is transmitted by ticks. Originally discovered by Romanian bacteriologist Victor Babeș in 1888; over 100 species of Babesia have since been identified.

Theileria is a genus of parasites that belongs to the phylum Apicomplexa, and is closely related to Plasmodium. Two Theileria species, T. annulata and T. parva, are important cattle parasites. T. annulata causes tropical theileriosis and T. parva causes East Coast fever. Theileria species are transmitted by ticks. The genomes of T. orientalis Shintoku, Theileria equi WA, Theileria annulata Ankara and Theileria parva Muguga have been sequenced and published.

Babesia microti is a parasitic blood-borne piroplasm transmitted by deer ticks. B. microti is responsible for the disease babesiosis, a malaria-like zoonosis which causes fever, hemolytic anemia caused by hemolysis, and enlarged spleen.

East Coast fever, also known as theileriosis, is a disease of cattle which occurs in Africa and is caused by the protozoan parasite Theileria parva. The primary vector which spreads T. parva between cattle is a tick, Rhipicephalus appendiculatus. East Coast fever is of major economic importance to livestock farmers in Africa, killing at least one million cattle each year. The disease occurs in Burundi, Democratic Republic of Congo, Kenya, Malawi, Mozambique, Rwanda, South Sudan, Tanzania, Uganda, Zimbabwe, Zambia. In 2003, East Coast fever was introduced to Comoros by cattle imported from Tanzania. It has been eradicated in South Africa.
Tropical theileriosis or Mediterranean theileriosis is a theileriosis of cattle from the Mediterranean and Middle East area, from Morocco to Western parts of India and China. It is a tick-borne disease, caused by Theileria annulata. The vectors are ticks of the genera Hyalomma and Rhipicephalus.
Hematozoa is a subclass of blood parasites of the Apicomplexa clade. Well known examples include the Plasmodium spp. which cause malaria in humans and Theileria which causes theileriosis in cattle. A large number of species are known to infect birds and are transmitted by insect vectors. The pattern in which Haematozoa infect a host cell depends on the genera of the blood parasite. Plasmodium and Leucozytozoon displace the nucleus of the host cell so that the parasite can take control of the cell where as Hemoproteus completely envelops the nucleus in a host cell.
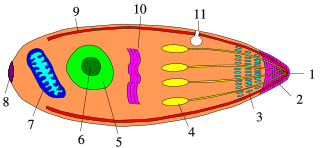
Apicomplexans, a group of intracellular parasites, have life cycle stages that allow them to survive the wide variety of environments they are exposed to during their complex life cycle. Each stage in the life cycle of an apicomplexan organism is typified by a cellular variety with a distinct morphology and biochemistry.

Babesia bovis is an Apicomplexan single-celled parasite of cattle which occasionally infects humans. The disease it and other members of the genus Babesia cause is a hemolytic anemia known as babesiosis and colloquially called Texas cattle fever, redwater or piroplasmosis. It is transmitted by bites from infected larval ticks of the order Ixodida. It was eradicated from the United States by 1943, but is still present in Mexico and much of the world's tropics. The chief vector of Babesia species is the southern cattle fever tick Rhipicephalus microplus.

Ticks of domestic animals directly cause poor health and loss of production to their hosts. Ticks also transmit numerous kinds of viruses, bacteria, and protozoa between domestic animals. These microbes cause diseases which can be severely debilitating or fatal to domestic animals, and may also affect humans. Ticks are especially important to domestic animals in tropical and subtropical countries, where the warm climate enables many species to flourish. Also, the large populations of wild animals in warm countries provide a reservoir of ticks and infective microbes that spread to domestic animals. Farmers of livestock animals use many methods to control ticks, and related treatments are used to reduce infestation of companion animals.
Cytauxzoon is a genus of parasitic alveolates in the phylum Apicomplexa. The name is derived from the Greek meaning an increase in the number of cells in an animal.

Babesia canis is a parasite that infects red blood cells and can lead to anemia. This is a species that falls under the overarching genus Babesia. It is transmitted by the brown dog tick and is one of the most common piroplasm infections. The brown dog tick is adapted to warmer climates and is found in both Europe and the United States, especially in shelters and greyhound kennels. In Europe, it is also transmitted by Dermacentor ticks with an increase in infections reported due to people traveling with their pets.

Sarcocystis neurona is primarily a neural parasite of horses and its management is of concern in veterinary medicine. The protozoan Sarcocystis neurona is a protozoan of single celled character and belongs to the family Sarcocystidae, in a group called coccidia. The protozoan, S. neurona, is a member of the genus Sarcocystis, and is most commonly associated with equine protozoal myeloencephalitis (EPM). S. neurona can be easily cultivated and genetically manipulated, hence its common use as a model to study numerous aspects of cell biology.

Quartan fever is one of the four types of malaria which can be contracted by humans.













