
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While the activation energy must be overcome to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually used with internal combustion engines fueled by gasoline or diesel, including lean-burn engines, and sometimes on kerosene heaters and stoves.
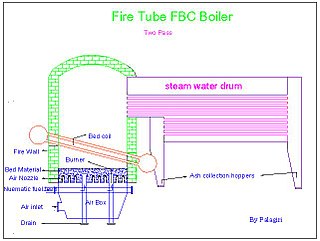
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

A recuperator is a special purpose counter-flow energy recovery heat exchanger positioned within the supply and exhaust air streams of an air handling system, or in the exhaust gases of an industrial process, in order to recover the waste heat. Generally, they are used to extract heat from the exhaust and use it to preheat air entering the combustion system. In this way they use waste energy to heat the air, offsetting some of the fuel, and thereby improve the energy efficiency of the system as a whole.
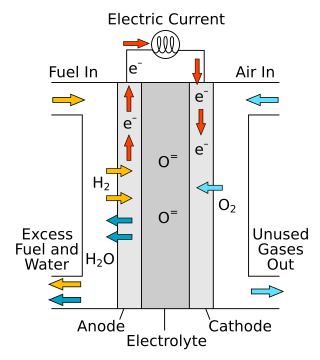
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
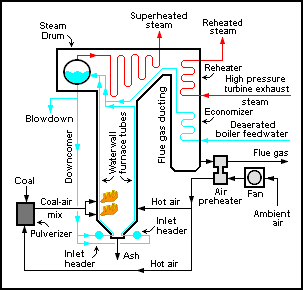
An air preheater is any device designed to heat air before another process (for example, combustion in a boiler With the primary objective of increasing the thermal efficiency of the process. They may be used alone or to replace a recuperative heat system or to replace a steam coil.

A diesel particulate filter (DPF) is a device designed to remove diesel particulate matter or soot from the exhaust gas of a diesel engine.

Cement kilns are used for the pyroprocessing stage of manufacture of portland and other types of hydraulic cement, in which calcium carbonate reacts with silica-bearing minerals to form a mixture of calcium silicates. Over a billion tonnes of cement are made per year, and cement kilns are the heart of this production process: their capacity usually defines the capacity of the cement plant. As the main energy-consuming and greenhouse-gas–emitting stage of cement manufacture, improvement of kiln efficiency has been the central concern of cement manufacturing technology. Emissions from cement kilns are a major source of greenhouse gas emissions, accounting for around 2.5% of non-natural carbon emissions worldwide.
Wet oxidation is a form of hydrothermal treatment. It is the oxidation of dissolved or suspended components in water using oxygen as the oxidizer. It is referred to as "Wet Air Oxidation" (WAO) when air is used. The oxidation reactions occur in superheated water at a temperature above the normal boiling point of water (100 °C), but below the critical point (374 °C).
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.

A waste heat recovery unit (WHRU) is an energy recovery heat exchanger that transfers heat from process outputs at high temperature to another part of the process for some purpose, usually increased efficiency. The WHRU is a tool involved in cogeneration. Waste heat may be extracted from sources such as hot flue gases from a diesel generator, steam from cooling towers, or even waste water from cooling processes such as in steel cooling.

Ventilation air methane thermal oxidizers (or VAMTOX) are a type of processing equipment used for greenhouse gas abatement related to underground mining operations that destroys gaseous methane at a high temperature.
A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce califaction (heat). When the catalyst, fuel, and oxygen combine together they react at a low enough temperatures that a flame is not produced. This process keeps repeating itself until either oxygen or the fuel source is taken out of the equation.

Yurii Shaevich Matros was a scientist in the field of chemical engineering, known for his achievement in the theory and practice of heterogeneous catalytic processes. He is acknowledged as a “Godfather” of realization of catalytic processes in forced unsteady state conditions. Matros developed a catalytic reactor with periodic changes of direction of flow rate in packed bed of catalyst. This reactor is widely known in scientific and applied literature as an example of an application of developed theory of forced unsteady processes. Yurii Matros possessed a full doctoral degree of science and was a professor.
Lower-temperature fuel cell types such as the proton exchange membrane fuel cell, phosphoric acid fuel cell, and alkaline fuel cell require pure hydrogen as fuel, typically produced from external reforming of natural gas. However, fuels cells operating at high temperature such as the solid oxide fuel cell (SOFC) are not poisoned by carbon monoxide and carbon dioxide, and in fact can accept hydrogen, carbon monoxide, carbon dioxide, steam, and methane mixtures as fuel directly, because of their internal shift and reforming capabilities. This opens up the possibility of efficient fuel cell-based power cycles consuming solid fuels such as coal and biomass, the gasification of which results in syngas containing mostly hydrogen, carbon monoxide and methane which can be cleaned and fed directly to the SOFCs without the added cost and complexity of methane reforming, water gas shifting and hydrogen separation operations which would otherwise be needed to isolate pure hydrogen as fuel. A power cycle based on gasification of solid fuel and SOFCs is called an Integrated Gasification Fuel Cell (IGFC) cycle; the IGFC power plant is analogous to an integrated gasification combined cycle power plant, but with the gas turbine power generation unit replaced with a fuel cell power generation unit. By taking advantage of intrinsically high energy efficiency of SOFCs and process integration, exceptionally high power plant efficiencies are possible. Furthermore, SOFCs in the IGFC cycle can be operated so as to isolate a carbon dioxide-rich anodic exhaust stream, allowing efficient carbon capture to address greenhouse gas emissions concerns of coal-based power generation.

A fluidized bed concentrator (FBC) is an industrial process for the treatment of exhaust air. The system uses a bed of activated carbon beads to adsorb volatile organic compounds (VOCs) from the exhaust gas. Differently from the fixed-bed or carbon rotor concentrators, the FBC system forces the VOC-laden air through several perforated steel trays, increasing the velocity of the air and allowing the sub-millimeter carbon beads to fluidize, or behave as if suspended in a liquid. This increases the surface area of the carbon-gas interaction, making it more effective at capturing VOCs.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.




















