
The visible spectrum is the band of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light. The optical spectrum is sometimes considered to be the same as the visible spectrum, but some authors define the term more broadly, to include the ultraviolet and infrared parts of the electromagnetic spectrum as well, known collectively as optical radiation.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.
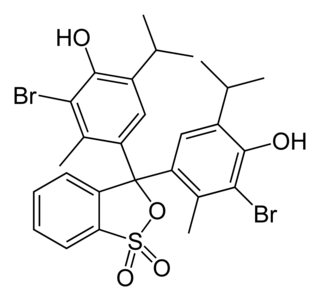
Bromothymol blue is a pH indicator. It is mostly used in applications that require measuring substances that would have a relatively neutral pH. A common use is for measuring the presence of carbonic acid in a liquid. It is typically sold in solid form as the sodium salt of the acid indicator.

Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
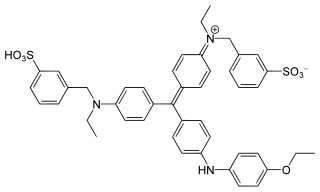
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.

Bromophenol blue, albutest is used as a pH indicator, an electrophoretic color marker, and a dye. It can be prepared by slowly adding excess bromine to a hot solution of phenolsulfonphthalein in glacial acetic acid.
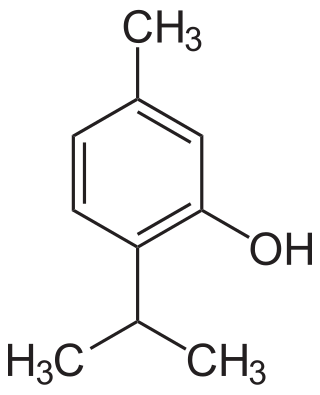
Thymol, C10H14O, is a natural monoterpenoid phenol derivative of p-Cymene, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris, ajwain, and various other plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris. Thymol is only slightly soluble in water at neutral pH, but it is extremely soluble in alcohols and other organic solvents. It is also soluble in strongly alkaline aqueous solutions due to deprotonation of the phenol. Its dissociation constant (pKa) is 10.59±0.10. Thymol absorbs maximum UV radiation at 274 nm.

Thymolphthalein is a phthalein dye used as an acid–base (pH) indicator. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The molar extinction coefficient for the blue thymolphthalein dianion is 38,000 M−1 cm−1 at 595 nm.

Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the pKa of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity.

A universal indicator is a pH indicator made of a solution of several compounds that exhibit various smooth colour changes over a wide range pH values to indicate the acidity or alkalinity of solutions. A universal indicator can be in paper form or present in a form of a solution.

Phenol red is a pH indicator frequently used in cell biology laboratories.
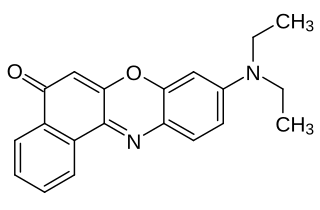
Nile red is a lipophilic stain. Nile red stains intracellular lipid droplets yellow. In most polar solvents, Nile red will not fluoresce; however, when in a lipid-rich environment, it can be intensely fluorescent, with varying colors from deep red to strong yellow-gold emission. The dye is highly solvatochromic and its emission and excitation wavelength both shift depending on solvent polarity and in polar media will hardly fluoresce at all.

A chromophore is a molecule which absorbs light at a particular wavelength and emits color as a result. Chromophores are commonly referred to as colored molecules for this reason. The word is derived from Ancient Greek χρῶμᾰ (chroma) 'color' and -φόρος (phoros) 'carrier of'. Many molecules in nature are chromophores, including chlorophyll, the molecule responsible for the green colors of leaves. The color that is seen by our eyes is that of the light not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore indicates a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light.
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV.

Dansyl chloride or 5-(dimethylamino)naphthalene-1-sulfonyl chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. It can also be made to react with secondary amines. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing and amino acid analysis. Dansyl chloride may also be denoted DNSC. Likewise, a similar derivative, dansyl amide is known as DNSA.

Brooker's merocyanine is an organic dye belonging to the class of merocyanines.
The near-infrared (NIR) window defines the range of wavelengths from 650 to 1350 nanometre (nm) where light has its maximum depth of penetration in tissue. Within the NIR window, scattering is the most dominant light-tissue interaction, and therefore the propagating light becomes diffused rapidly. Since scattering increases the distance travelled by photons within tissue, the probability of photon absorption also increases. Because scattering has weak dependence on wavelength, the NIR window is primarily limited by the light absorption of blood at short wavelengths and water at long wavelengths. The technique using this window is called NIRS. Medical imaging techniques such as fluorescence image-guided surgery often make use of the NIR window to detect deep structures.

Thiosulfate–citrate–bile salts–sucrose agar, or TCBS agar, is a type of selective agar culture plate that is used in microbiology laboratories to isolate Vibrio species. TCBS agar is highly selective for the isolation of V. cholerae and V. parahaemolyticus as well as other Vibrio species. Apart from TCBS agar, other rapid testing dipsticks like immunochromatographic dipstick is also used in endemic areas such as Asia, Africa and Latin America. Though, TCBS agar study is required for confirmation. This becomes immensely important in cases of gastroenteritis caused by campylobacter species, whose symptoms mimic that of cholera. Since no yellow bacterial growth is observed in case of campylobacter species on TCBS agar, chances of incorrect diagnosis can be rectified. TCBS agar contains high concentrations of sodium thiosulfate and sodium citrate to inhibit the growth of Enterobacteriaceae. Inhibition of gram-positive bacteria is achieved by the incorporation of ox gall, which is a naturally occurring substance containing a mixture of bile salts and sodium cholate, a pure bile salt. Sodium thiosulfate also serves as a sulfur source and its presence, in combination with ferric citrate, allows for the easy detection of hydrogen sulfide production. Saccharose (sucrose) is included as a fermentable carbohydrate for metabolism by Vibrio species. The alkaline pH of the medium enhances the recovery of V. cholerae and inhibits the growth of others. Thymol blue and bromothymol blue are included as indicators of pH changes.
In chemistry, the molar absorption coefficient or molar attenuation coefficient is a measurement of how strongly a chemical species absorbs, and thereby attenuates, light at a given wavelength. It is an intrinsic property of the species. The SI unit of molar absorption coefficient is the square metre per mole, but in practice, quantities are usually expressed in terms of M−1⋅cm−1 or L⋅mol−1⋅cm−1. In older literature, the cm2/mol is sometimes used; 1 M−1⋅cm−1 equals 1000 cm2/mol. The molar absorption coefficient is also known as the molar extinction coefficient and molar absorptivity, but the use of these alternative terms has been discouraged by the IUPAC.






















