 | |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.016.026 |
| EC Number |
|
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
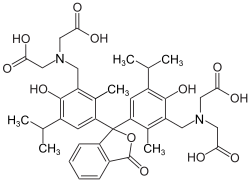
| C38H44N2O12 | |
| Molar mass | 720.772 g·mol−1 |
| Appearance | white crystalline powder |
| Melting point | 191 °C (376 °F; 464 K) |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Thymolphthalexone is a chemical compound from the group of iminodiacetic acid derivatives of thymolphthalein. [3] Its chemical formula is C38H44N2O12.
Contents
This is a metallochromic indicator widely used in complexometric titrations, particularly for the determination of transition metals. The compound features a thymolphthalein-derived core linked to aminopolycarboxylic acid functional groups. This hybrid architecture grants the compound the ability to preferentially bind specific metal ions through coordinated interactions.