Timeless homologs
| timeout | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | tim-2 | ||||||
| UniProt | Q8INH7 | ||||||
| |||||||
| Timeout, C-terminal (PAB) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 Human TIMELESS PAB | |||||||||||
| Identifiers | |||||||||||
| Symbol | TIMELESS_C | ||||||||||
| Pfam | PF05029 | ||||||||||
| InterPro | IPR006906 | ||||||||||
| |||||||||||
Drosophila homolog
The timeless gene is an essential component of the molecular circadian clock in Drosophila. [3] It acts as part of an autoregulatory feedback loop in conjunction with the period (per) gene product as noted in collaborative studies performed by the labs of Michael W. Young and Amita Sehgal. [7] Further studies by the labs of Young, Sehgal, Charles Weitz, and Michael Rosbash indicated that timeless protein (TIM) and period protein (PER) form a heterodimer that exhibits circadian rhythms in wild type Drosophila. [8] [9] Researchers in Rosbash's lab also showed that tim mRNA levels and TIM protein levels have circadian rhythms that are similar to those of the period (per) mRNA and its product. [8] [10] [11] Experiments done jointly by the Weitz, Young, and Sehgal labs using yeast 2-hybrid proved that TIM directly binds with PER. [12] During the early evening, PER and TIM dimerize and accumulate. Late at night, the dimer travels into the nucleus to inhibit per and tim transcription. In 1996, the teams of Sehgal, Edery, and Young found that exposure to light leads to the degradation of TIM and subsequently PER. [1] [11] [13]
The PER/TIM heterodimer negatively regulates transcription of period (per) and timeless (tim) genes. Within this negative feedback loop, first the PER/TIM heterodimers form in the cytoplasm, accumulate, and then translocate to the nucleus. [14] The complex then blocks the positive transcription factors clock (CLK) and cycle (CYC), thereby repressing the transcription of per.
As part of the circadian clock, timeless is essential for entrainment to light-dark (LD) cycles. The typical period length of free-running Drosophila is 23.9 hours, requiring adaptations to the 24-hour environmental cycle. [15] Adaptation first begins with exposure to light. This process leads to the rapid degradation of the TIM protein, allowing organisms to entrain at dawn to environmental cycles. [16]

In light-dark cycles, TIM protein level decreases rapidly in late night/early morning, followed by the similar but more gradual changes in PER protein level. TIM degradation is independent of per and its protein, and releases PER from the PER/TIM complex. [8] In some cell types, the photoreceptor protein cryptochrome (CRY) physically associates with TIM and helps regulate light-dependent degradation. CRY is activated by blue light, which binds to TIM and tags it for degradation. [17] This ends the PER/TIM repression of the CLK/CYC-mediated transcription of per and tim genes, allowing per and tim mRNA to be produced to restart the cycle. [8]
This mechanism allows entrainment of flies to environmental light cues. When Drosophila receive light inputs in the early subjective night, light-induced TIM degradation causes a delay in TIM accumulation, which creates a phase delay. [17] When light inputs are received in the late subjective night, a light pulse causes TIM degradation to occur earlier than under normal conditions, leading to a phase advance. [17]
In Drosophila, the negative regulator PER, from the PER/TIM complex, is eventually degraded by a casein kinase-mediated phosphorylation cycle, allowing fluctuations in gene expression according to environmental cues. These proteins mediate the oscillating expression of the transcription factor VRILLE (VRI), which is required for behavioral rhythmicity, per and tim expression, and accumulation of PDF (pigment-dispersing factor). [16]
Gryllus bimaculatus (two-spotted cricket) homolog
Timeless does not appear to be essential for oscillation of the circadian clock for all insects. In wild type Gryllus bimaculatus,tim mRNA shows rhythmic expression in both LD and DD (dark-dark cycles) similar to that of per, peaking during the subjective night. When injected with tim double-stranded RNA (dstim), tim mRNA levels were significantly reduced and its circadian expression rhythm was eliminated. After the dstim treatment, however, adult crickets showed a clear locomotor rhythm in constant darkness, with a free-running period significantly shorter than that of control crickets injected with Discosoma sp. Red2 (DsRed2) dsRNA. These results suggest that in the cricket, tim plays some role in fine-tuning of the free-running period but may not be essential for oscillation of the circadian clock. [5]
Mammalian homolog
In 1998, researchers identified a mouse homolog and a human homolog of the Drosophilatimeless gene. [18] The exact role of TIM in mammals is still unclear,. Recent work on the mammalian timeless (mTim) in mice has suggested that the gene may not play the same essential role in mammals as in Drosophila as a necessary function of the circadian clock. [19] While Tim is expressed in the Suprachiasmatic Nucleus (SCN) which is thought to be the primary oscillator in humans, its transcription does not oscillate rhythmically in constant conditions, and the TIM protein remains in the nucleus. [19] [20]

However, mTim is shown to be necessary for embryonic development in mice, indicating a different gene function than in Drosophila. This suggests a divergence between mammalian clocks and the Drosophila clock. [19] Moreover, mammalian tim is more orthologous to the Tim-2 (Timeout) paralog of the DrosophilaTimeless gene than the actual gene itself. [21] Like tim-2, the mammalian orthologs has a C-terminal PARP1-binding (PAB) domain. The complex they from promotes homologous recombination DNA repair. [22]
The timeless protein is thought to directly connect the cell cycle with the circadian rhythm in mammals. In this model. referred to as a “direct coupling,” [23] the two cycles share a key protein whose expression exhibits a circadian pattern. The essential role of Tim in Drosophila in creating circadian rhythm is accomplished by Cry in mammals. In mammals, Cry and Per transcription is activated by the CLOCK/BMAL1 complex, and repressed by the PER/CRY complex. [24]
Humans
| timeless homolog (Human) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | TIMELESS | ||||||
| Alt. symbols | hTIM | ||||||
| NCBI gene | 8914 | ||||||
| HGNC | 11813 | ||||||
| OMIM | 603887 | ||||||
| RefSeq | NM_003920 | ||||||
| UniProt | Q9UNS1 | ||||||
| Other data | |||||||
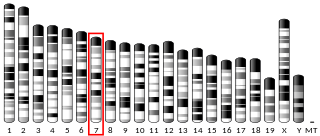
| Locus | Chr. 12 q12-q13 | ||||||
| |||||||
The human timeless protein (hTIM) has been shown to be required for the production of electrical oscillations output by the suprachiasmatic nucleus (SCN), the major clock governing all tissue-specific circadian rhythms of the body. [25] This protein also interacts with the products of major clock genes CLOCK, BMAL, PER1, PER2 and PER3.
Sancar and colleagues investigated whether hTIM played a similar role to orthologs in C. elegans and types of yeast, which are known to play important roles in the cell cycle. [23] Their experiments suggested that hTIM plays an integral role in the G2/M and intra-S cell cycle checkpoints. [23] With respect to the G2/M checkpoint, hTIM binds to the ATRIP subunit on ATR – a protein kinase sensitive to DNA damage. This binding between hTIM and ATR then leads to the phosphorylation of Chk1, resulting in cell cycle arrest or apoptosis. [23] This process serves as an important control to stop the proliferation of cells with DNA damage prior to mitotic division. The role of hTIM in the intra-S checkpoint is less clear at the molecular level; however, down-regulation of hTIM leads to an increase in the rate of generation of replication forks – even in the presence of DNA damage and other regulatory responses. [23]