
Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.

Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection people often have mild or no symptoms. Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs. The virus persists in the liver in about 75% to 85% of those initially infected. Early on chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.

Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled.

The hepatitis C virus (HCV) is a small, enveloped, positive-sense single-stranded RNA virus of the family Flaviviridae. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.

Taribavirin is an antiviral drug in Phase III human trials, but not yet approved for pharmaceutical use. It is a prodrug of ribavirin, active against a number of DNA and RNA viruses. Taribavirin has better liver-targeting than ribavirin, and has a shorter life in the body due to less penetration and storage in red blood cells. It is expected eventually to be the drug of choice for viral hepatitis syndromes in which ribavirin is active. These include hepatitis C and perhaps also hepatitis B and yellow fever.

Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections. It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza. Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths; evidence as of 2014 suggested that it possesses efficacy in treating a number of viral infections as well.

Hepatitis B is an infectious disease caused by the hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection. Many people have no symptoms during the initial infection. In acute infection, some may develop a rapid onset of sickness with vomiting, yellowish skin, tiredness, dark urine, and abdominal pain. Often these symptoms last a few weeks and rarely does the initial infection result in death. It may take 30 to 180 days for symptoms to begin. In those who get infected around the time of birth 90% develop chronic hepatitis B while less than 10% of those infected after the age of five do. Most of those with chronic disease have no symptoms; however, cirrhosis and liver cancer may eventually develop. Cirrhosis or liver cancer occur in about 25% of those with chronic disease.

Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without trans-membrane helices.

Hepatitis B virus (HBV), is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
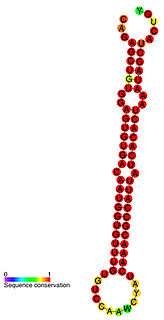
miR-122 is a miRNA that is conserved among vertebrate species. miR-122 is not present in invertebrates, and no close paralogs of miR-122 have been detected. miR-122 is highly expressed in the liver, where it has been implicated as a regulator of fatty-acid metabolism in mouse studies. Reduced miR-122 levels are associated with hepatocellular carcinoma. miR-122 also plays an important positive role in the regulation of hepatitis C virus replication.

FGI-104 is the name of an experimental broad-spectrum antiviral drug, with activity against a range of viruses including Hepatitis B, Hepatitis C, HIV, Ebola virus, and Venezuelan equine encephalitis virus.
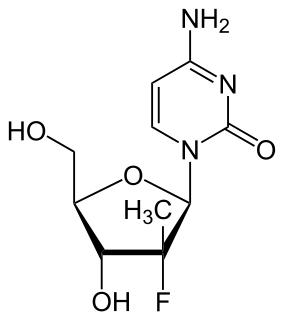
PSI-6130 is an experimental treatment for hepatitis C. PSI-6130 is a member of a class of antiviral drugs known as nucleoside polymerase inhibitors that was created by chemist Jeremy L. Clark. Specifically, PSI-6130 inhibits the hepatitis C virus RNA dependant RNA polymerase called NS5B.

Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV). It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication. Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK. The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4's (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.

Non-structural protein 5B (NS5B) inhibitors are a class of direct acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes – nucleoside active site inhibitors (NIs), non nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct acting NS5B inhibitors does not takes place cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.
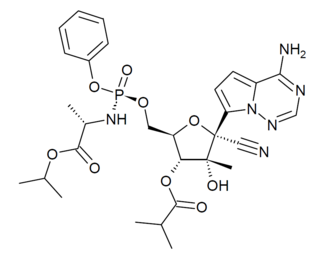
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
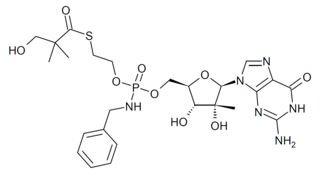
IDX-184 is an antiviral drug which was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor. While it showed reasonable effectiveness in early clinical trials it did not progress past Phase IIb. However research using this drug has continued as it shows potentially useful activity against other emerging viral diseases such as Zika virus, and coronaviruses including MERS, and SARS-CoV-2.

Merimepodib (VX-497) is a drug which acts as an inhibitor of the enzyme inosine monophosphate dehydrogenase, which is required for the synthesis of nucleotide bases containing guanine. This consequently inhibits synthesis of DNA and RNA, and results in antiviral and immunosuppressive effects. It progressed as far as Phase 2b human clinical trials against Hepatitis C but showed only modest benefits in comparison to existing treatments, however it continues to be researched, and also shows activity against other viral diseases such as Zika virus and foot and mouth disease virus.
















