
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.
Keratolytic therapy is a type of medical treatment to remove warts, calluses and other lesions in which the epidermis produces excess skin. In this therapy, acidic topical medicines, such as Whitfield's ointment or Jessner's solution, are applied to the lesion in order to thin the skin on and around it. This therapy causes the outer layer of the skin to loosen and shed.
In organic chemistry, the chloroacetic acids (systematic name chloroethanoic acids) are three related chlorocarbon carboxylic acids:

Trichloroacetic acid is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are called trichloroacetates.
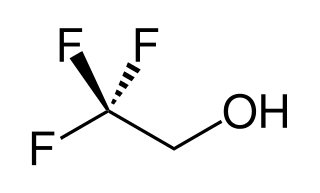
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol.

Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a haloacetic acid, with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms. It is a colorless liquid with a vinegar-like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant, Ka, that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes.
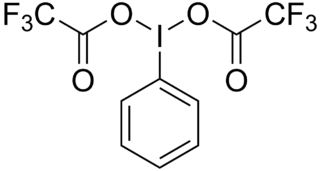
(Bis iodo)benzene, C
6H
5I(OCOCF
3)
2, is a hypervalent iodine compound used as a reagent in organic chemistry. It can be used to carry out the Hofmann rearrangement under acidic conditions.

Fluoroacetic acid is a organofluorine compound with formula FCH2CO2H. It is a colorless solid that is noted for its relatively high toxicity. The conjugate base, fluoroacetate occurs naturally in at least 40 plants in Australia, Brazil, and Africa. It is one of only five known organic fluorine-containing natural products.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.

Haloacetic acids or HAAs are carboxylic acids in which one or more halogen atoms take the place of hydrogen atoms in the methyl group of acetic acid. Those acids have a general chemical formula X1X2X3C−CO2H, where X is hydrogen or halogen, and at least one X is a halogen. In a monohaloacetic acid, a single halogen replaces a hydrogen atom: for example, in bromoacetic acid. Further substitution of hydrogen atoms with halogens can occur, as in dichloroacetic acid and trichloroacetic acid.

Sodium trichloroacetate is a chemical compound with a formula of CCl3CO2Na. It is used to increase sensitivity and precision during transcript mapping. It was previously used as an herbicide starting in the 1950s but regulators removed it from the market in the late 1980s and early 1990s.
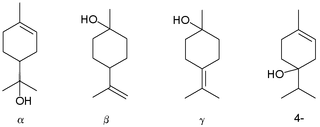
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil. Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. β- and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent.

Cefroxadine is a cephalosporin antibiotic. It is structurally related to cefalexin, and both drugs share a similar spectrum of activity.
Chloroformic acid is a chemical compound with the formula ClCO2H. It is the single acyl-halide derivative of carbonic acid. Chloroformic acid is also structurally related to formic acid, in a way that the non-acidic hydrogen of formic acid is replaced by chlorine. Despite the similar name, it is very different from chloroform. It is described as unstable.

Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride.
Perfluoroalkyl carboxylic acids (PFCAs), or perfluorocarboxylic acids are compounds of the formula CnF(2n+1)CO2H that belong to the class of per- and polyfluoroalkyl substances. The simplest example is trifluoroacetic acid. These compounds are organofluorine analogues of ordinary carboxylic acids, but they are stronger by several pKa units and they exhibit great hydrophobic character. Perfluoroalkyl dicarboxylic acids (PFdiCAs) are also known, e.g. C2F4(CO2H)2.

Topilutamide, known more commonly as fluridil and sold under the brand name Eucapil, is an antiandrogen medication which is used in the treatment of pattern hair loss in men and women. It is used as a topical medication and is applied to the scalp. Topilutamide belongs to a class of molecules known as perfluoroacylamido-arylpropanamides.

Trifluoroperacetic acid is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.

Sodium trifluoroacetate is a chemical compound with a formula of CF3CO2Na. It is the sodium salt of trifluoroacetic acid. It is used as a source of trifluoromethylations.

Tin(IV) nitrate is a salt of tin with nitric acid. It is a volatile white solid, subliming at 40 °C under a vacuum. Unlike other nitrates, it reacts with water to produce nitrogen dioxide.

















