
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. Rarer still, they may contain elements such as phosphorus, chlorine, and bromine.
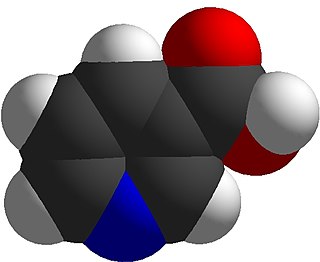
Niacin, also known as nicotinic acid, is an organic compound and a vitamer of vitamin B3, an essential human nutrient. It is produced by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds. Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness. Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.

Fenugreek is an annual plant in the family Fabaceae, with leaves consisting of three small obovate to oblong leaflets. It is cultivated worldwide as a semiarid crop. Its leaves and seeds are common ingredients in dishes from the Indian subcontinent, and have been used as a culinary ingredient since ancient times. Its use as a food ingredient in small quantities is safe.

Coniine is a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis. The biosynthesis of coniine contains as its penultimate step the non-enzymatic cyclisation of 5-oxooctylamine to γ-coniceine, a Schiff base differing from coniine only by its carbon-nitrogen double bond in the ring. This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (R)-enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in organic chemistry history as being the first of the important class of alkaloids to be synthesized, by Albert Ladenburg in 1886, and it has been synthesized in the laboratory in a number of unique ways through to modern times.
Piperine, possibly along with its isomer chavicine, is the compound responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine.
The Fischer oxazole synthesis is a chemical synthesis of an oxazole from a cyanohydrin and an aldehyde in the presence of anhydrous hydrochloric acid. This method was discovered by Emil Fischer in 1896. The cyanohydrin itself is derived from a separate aldehyde. The reactants of the oxazole synthesis itself, the cyanohydrin of an aldehyde and the other aldehyde itself, are usually present in equimolar amounts. Both reactants usually have an aromatic group, which appear at specific positions on the resulting heterocycle.
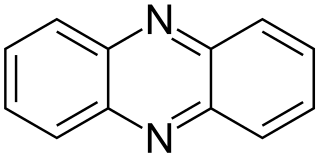
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.

Arecoline is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm. It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy along with mild feelings of euphoria and relaxation.
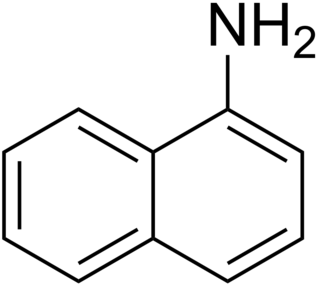
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer. It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.

Methyllycaconitine (MLA) is a diterpenoid alkaloid found in many species of Delphinium (larkspurs). In common with many other diterpenoid alkaloids, it is toxic to animals, although the acute toxicity varies with species. Methyllycaconitine was identified one of the principal toxins in larkspurs responsible for livestock poisoning in the mountain rangelands of North America. Methyllycaconitine has been explored as a possible therapeutic agent for the treatment of spastic paralysis, and it has been shown to have insecticidal properties. It has become an important molecular probe for studying the pharmacology of the nicotinic acetylcholine receptor.
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic mixture into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution.

Free base is a descriptor for the neutral form of an amine commonly used in reference to illicit drugs. The amine is often an alkaloid, such as nicotine, cocaine, morphine, and ephedrine, or derivatives thereof. Freebasing is a more efficient method of self-administering alkaloids via the smoking route.
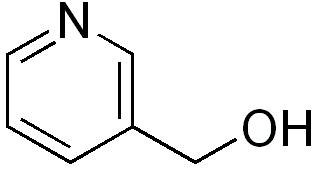
Nicotinyl alcohol (pyridylcarbinol) is a niacin derivative used as a hypolipidemic agent and as a vasodilator. It causes flushing and may decrease blood pressure.

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.

Alstonia constricta, commonly known as quinine bush or bitterbark, is an Australian endemic shrub or small tree of the family Apocynaceae.
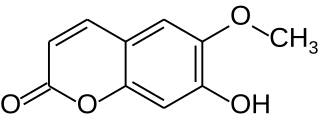
Scopoletin is a coumarin found in the root of plants in the genus Scopolia such as Scopolia carniolica and Scopolia japonica, in chicory, in Artemisia scoparia, in the roots and leaves of stinging nettle, in the passion flower, in Brunfelsia, in Viburnum prunifolium, in Solanum nigrum, in Datura metel, in Mallotus resinosus, or and in Kleinhovia hospita. It can also be found in fenugreek, vinegar, some whiskies or in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
1,4-butane sultone is a six-membered δ-sultone and the cyclic ester of 4-hydroxybutanesulfonic acid. As a sulfo-alkylating agent, 1,4-butanesultone is used to introduce the sulfobutyl group (–(CH2)4–SO3−) into hydrophobic compounds possessing nucleophilic functional groups, for example hydroxy groups (as in the case of β-cyclodextrin) or amino groups (as in the case of polymethine dyes). In such, the sulfobutyl group is present as neutral sodium salt and considerably increases the water solubility of the derivatives.
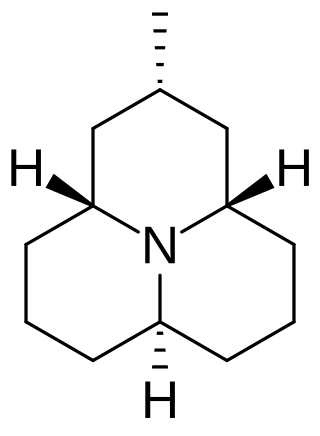
Precoccinelline is an alkaloid produced by the Coccinella septempunctata, also known as the seven-spot ladybird. The alkaloid is released from the joints in C. septempunctata legs when it is provoked to deter predators such as ants or birds. It binds to both insect and mammalian nicotinic acetylcholine receptors, giving it use as an insecticide or as a therapy to treat drug dependence.

Bismuthyl is an inorganic oxygen-containing singly charged ion with the chemical formula BiO+, and is an oxycation of bismuth in the +3 oxidation state. Most often it is formed during the hydrolysis of trivalent bismuth salts, primarily nitrate, chloride and other halides. In chemical compounds, bismuthyl plays the role of a monovalent cation.

















