
Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale. Phthalic acid is one of three isomers of benzenedicarboxylic acid, the others being isophthalic acid and terephthalic acid.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
Polyamide-imides are either thermosetting or thermoplastic, amorphous polymers that have exceptional mechanical, thermal and chemical resistant properties. Polyamide-imides are used extensively as wire coatings in making magnet wire. They are prepared from isocyanates and TMA in N-methyl-2-pyrrolidone (NMP). A prominent distributor of polyamide-imides is Solvay Specialty Polymers, which uses the trademark Torlon.

An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
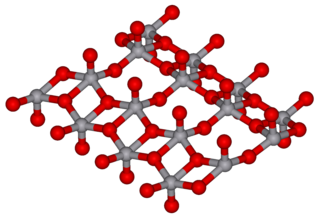
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemicals Ltd which is more economical and environmentally friendly.

Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Nucleophilic acyl substitution describes a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.
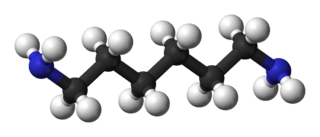
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.

Propionic anhydride is an organic compound with the formula (CH3CH2CO)2O. This simple acid anhydride is a colourless liquid. It is a widely used reagent in organic synthesis as well as for producing specialty derivatives of cellulose.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
The Yamaguchi esterification is the chemical reaction of an aliphatic carboxylic acid and 2,4,6-trichlorobenzoyl chloride to form a mixed anhydride which, upon reaction with an alcohol in the presence of stoichiometric amount of DMAP, produces the desired ester. It was first reported by Masaru Yamaguchi et al. in 1979.
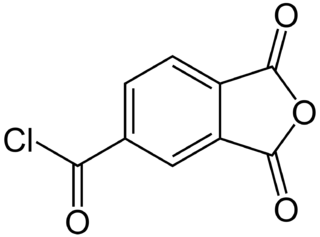
Trimellitic anhydride chloride is a chemical compound used to produce polyamide-imide plastic.
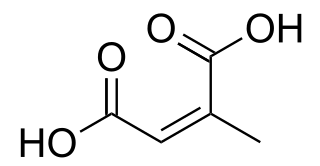
Citraconic acid is an organic compound with the formula CH3C2H(CO2H)2. It is a white solid. It is the cis-isomer of mesaconic acid. It is one of the pyrocitric acids formed upon the heating of citric acid. Citraconic acid can be produced, albeit inefficiently, by oxidation of xylene and methylbutanols. The acid displays the unusual property of spontaneously forming the anhydride, which, unlike maleic anhydride, is a liquid at room temperature.
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
Shiina esterification is an organic chemical reaction that synthesizes carboxylic esters from nearly equal amounts of carboxylic acids and alcohols by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic coupling method using Lewis acid, and, in 2002, a basic esterification using nucleophilic catalyst.

Tin(IV) nitrate is a salt of tin with nitric acid. It is a volatile white solid, subliming at 40 °C under a vacuum. Unlike other nitrates, it reacts with water to produce nitrogen dioxide.
















