
Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.

Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula [RhCl(PPh3)3], where 'Ph' denotes a phenyl group). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide.

Zeise's salt, potassium trichloro(ethylene)platinate(II) hydrate, is the chemical compound with the formula K[PtCl3(C2H4)]·H2O. The anion of this air-stable, yellow, coordination complex contains an η2-ethylene ligand. The anion features a platinum atom with a square planar geometry. The salt is of historical importance in the area of organometallic chemistry as one of the first examples of a transition metal alkene complex and is named for its discoverer, William Christopher Zeise.

Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula (Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.
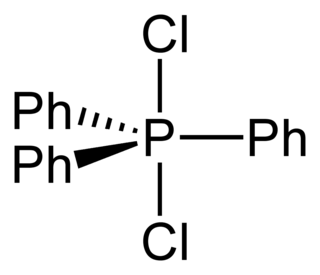
Triphenylphosphine dichloride, (C6H5)3PCl2, is a chlorinating agent widely used in organic chemistry. Applications include the conversion of alcohols and ethers to alkyl chlorides, the cleavage of epoxides to vicinal dichlorides and the chlorination of carboxylic acids to acyl chlorides.

Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Uranium pentachloride is an inorganic chemical compound composed of uranium in the +5 oxidation state and five chlorine atoms.
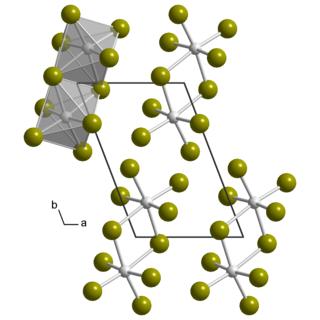
Uranium pentabromide is an inorganic chemical compound with the formula UBr5.

Potassium selenocyanate is the inorganic compound with the formula KSeCN. It is a hygroscopic white solid that is soluble in water, decomposing in air to red selenium and potassium cyanide. The compound has been characterized by X-ray crystallography, which confirms that it is a salt. The C-N and C-Se distances are 112 and 183 pm, respectively consistent with triple and single bonds.

Hydridotetrakis(triphenylphosphine)rhodium(I) is the coordination complex with the formula HRh[P(C6H5)3]4. It consists of a Rh(I) center complexed to four triphenylphosphine (PPh3) ligands and one hydride. The molecule has idealized C3v symmetry. The compound is a homogeneous catalyst for hydrogenation and related reactions. It is a yellow solid that dissolves in aromatic solvents.
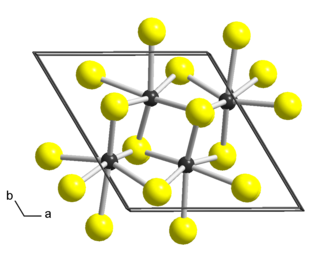
Rhenium disulfide is an inorganic compound of rhenium and sulfur with the formula ReS2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
The selenide iodides are chemical compounds that contain both selenide ions (Se2−) and iodide ions (I−) and one or metal atoms. They are in the class of mixed anion compounds or chalcogenide halides.
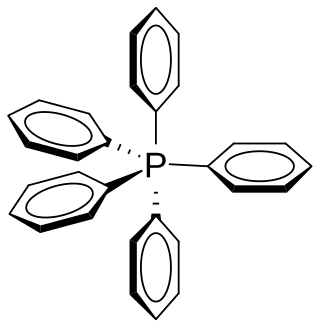
Pentaphenylphosphorus is an organic phosphorane containing five phenyl groups connected to a central phosphorus atom. The phosphorus atom is considered to be in the +5 oxidation state. The chemical formula could be written as P(C6H5)5 or Ph5P, where Ph represents the phenyl group. It was discovered and reported in 1949 by Georg Wittig.
















