In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
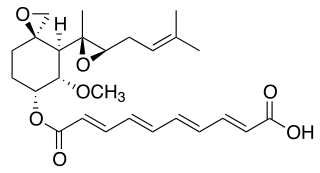
Fumagillin is a complex biomolecule and used as an antimicrobial agent. It was isolated in 1949 from the microbial organism Aspergillus fumigatus.
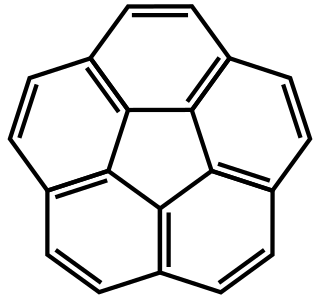
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:

Pentostatin is an anticancer chemotherapeutic drug.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.
Robert S. Coleman is an American chemistry professor and researcher. Coleman was a faculty member at both Ohio State University and the University of South Carolina. At Ohio State, he was on the faculty in the Department of Chemistry from 1996 to 2012, having moved to Ohio State as an associate professor from the University of South Carolina. At USC, Coleman taught as assistant professor from 1989 to 1995, and then as associate professor from 1995 to 1996. In 1996, he accepted a faculty position at Ohio State University to teach Organic Chemistry, where he was an associate professor from 1996 until 2000. He was promoted to full professor in 2000, teaching Organic Chemistry up until his retirement in 2012. He received his Ph.D. degree working with Professor Dale L. Boger, completing the first total synthesis of the antitumor agent CC-1065. He was subsequently an NIH postdoctoral fellow at Yale University with Professor Samuel J. Danishefsky, where he completed, the first total synthesis of the aglycone of the antitumor agent calicheamicin.

Annonacin is a chemical compound with toxic effects, especially in the nervous system, found in some fruits such as the paw paw, custard apples, soursop, and others from the family Annonaceae. It is a member of the class of compounds known as acetogenins. Annonacin-containing fruit products are regularly consumed throughout the West Indies for their traditional medicine uses.

Acetogenins are a class of polyketide natural products found in plants of the family Annonaceae. They are characterized by linear 32- or 34-carbon chains containing oxygenated functional groups including hydroxyls, ketones, epoxides, tetrahydrofurans and tetrahydropyrans. They are often terminated with a lactone or butenolide. Over 400 members of this family of compounds have been isolated from 51 different species of plants. Many acetogenins are characterized by neurotoxicity.

Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.

Dazoxiben is an orally active thromboxane synthase inhibitor. It has shown a significant clinical improvement in patients with Raynaud's syndrome.

Taspine is an alkaloid which acts as a potent acetylcholinesterase inhibitor and cicatrizant. It is found in various plants including Magnolia x soulangeana and Croton lechleri.

Anthony Gerard Martin Barrett FRS, FMedSci is a British chemist, and Sir Derek Barton Professor of Synthesis, Glaxo Professor of Organic Chemistry at Imperial College London. He is Director of the Wolfson Centre for Organic Chemistry in Medical Science. He was elected a fellow of the Royal Society in 1999 and Academy of Medical Sciences in 2003. He obtained a BSc as well as PhD from Imperial College London in 1973 and 1975 respectively.

Bullatacin is a bis(tetrahydrofuranoid) fatty acid lactone found in some fruits from Annonaceae family. It is a member of the class of compounds known as acetogenins.
Proline organocatalysis is the use of proline as an organocatalyst in organic chemistry. This theme is often considered the starting point for the area of organocatalysis, even though early discoveries went unappreciated. Modifications, such as MacMillan’s catalyst and Jorgensen's catalysts, proceed with excellent stereocontrol.
Peter Wipf is a distinguished university professor of chemistry at the University of Pittsburgh. His research interests focus on the total synthesis of natural products, the discovery of new transformations of strained molecules, and the development of new pharmaceuticals. He is a Fellow of the Royal Society of Chemistry (RSC), the American Association for the Advancement of Science (AAAS), and the American Chemical Society (ACS).
Mark S. Cushman is an American chemist, whose primary research is in the area of medicinal chemistry. He completed his pre-pharmacy studies at Fresno State College (now California State University, Fresno) in 1965. He then attended the University of California San Francisco (as a University of California Regents Scholar), earning a Pharm.D. in 1969 and a Ph.D. in Medicinal Chemistry in 1973. Thereafter, he performed postdoctoral training in the laboratory of George Büchi, Ph.D., at the Massachusetts Institute of Technology (MIT). There, his research focused on the discovery and development of new synthetic methodologies, and the isolation and structural characterization of mycotoxins from Aspergillus niger. In 1975, he joined the Department of Medicinal Chemistry and Molecular Pharmacology (at the time, Department of Medicinal Chemistry and Pharmacognosy) at Purdue University. From 1983 to 1984, Prof. Cushman was a Senior Fulbright Scholar at Munich Technical University working in the laboratory of Professor Adelbert Bacher. His sabbatical work dealt with the design and synthesis of probes to elucidate key aspects of the biosynthesis of riboflavin (vitamin B2). Currently he holds the rank of Distinguished Professor Emeritus of Medicinal Chemistry at Purdue University. He has mentored 40 graduate students, 59 postdoctoral researchers, and 5 visiting scholars. He has published 348 papers and holds 41 patents. His work has ~17,000 citations with an h-index of 69. His most cited papers had 471, 403, and 299 citations as of August 2021. He has made seminal contributions to the fields of synthetic and medicinal chemistry including the development of new synthetic methodologies, the synthesis of natural products, and the preparation of antivirals, antibacterials, and anticancer agents, and mechanism probes to understand the function of over thirty macromolecular targets. One of his main scientific contributions is the development of the indenoisoquinolines, molecules that inhibit the action of toposiomerase I (Top1) and stabilize the G-quadruplex in the Myc promoter. Three indenoisoquinolines designed and synthesized by his research group at Purdue University [indotecan (LMP 400), indimitecan (LMP 776), and LMP 744] demonstrated potent anticancer activity in vivo and have completed phase I clinical trials at the National Institutes of Health.
The Fiesselmann thiophene synthesis is a name reaction in organic chemistry that allows for the generation of 3-hydroxy-2-thiophenecarboxylic acid derivatives from α,β-acetylenic esters with thioglycolic acid and its derivatives under the presence of a base. The reaction was developed by Hans Fiesselmann in the 1950s.










