
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.
Thearubigins are polymeric polyphenols that are formed during the enzymatic oxidation and condensation of two gallocatechins with the participation of polyphenol oxidases during the fermentation reactions in black tea. Thearubigins are red in colour and are responsible for much of the staining effect of tea. Therefore, a black tea often appears red while a green or white tea has a much clearer appearance. The colour of a black tea, however, is affected by many other factors as well, such as the amount of theaflavins, another oxidized form of polyphenols.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

The color of wine is one of the most easily recognizable characteristics of wines. Color is also an element in wine tasting since heavy wines generally have a deeper color. The accessory traditionally used to judge the wine color was the tastevin, a shallow cup allowing one to see the color of the liquid in the dim light of a cellar. The color is an element in the classification of wines.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

The grape reaction product is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing. It is also found in aged red wines. Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts, especially in white wine production. The product can be recreated in model solutions.

Vitisin B is a natural phenol found in red wines. It is a pyranoanthocyanin.
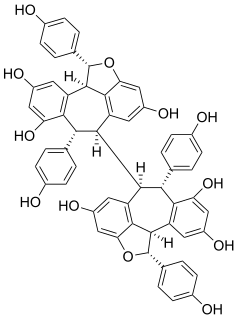
Hopeaphenol is a stilbenoid. It is a resveratrol tetramer. It has been first isolated from Dipterocarpaceae like Shorea ovalis. It has also been isolated from wines from North Africa.
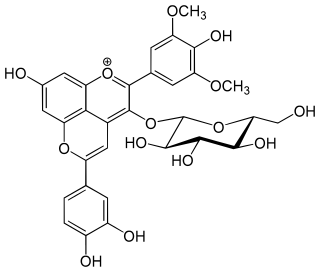
Pinotins are a type of pyranoanthocyanins, a class of phenolic compounds found in red wine. One such compound is pinotin A.

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4.

Pinotin A is a pinotin, a type of pyranoanthocyanins and a class of phenolic compounds found in red wine.
Proteins are present in wine. The most common proteins include thaumatin-like proteins and chitinases and have a role in the formation of turbidity (haze) especially visible in white wine. The quantity of haze forming is dependent on the quantity of phenolics in the wine.
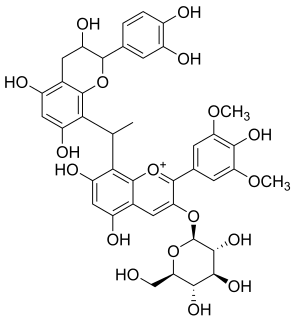
Malvidin glucoside-ethyl-catechin is a flavanol-anthocyanin adduct. Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.
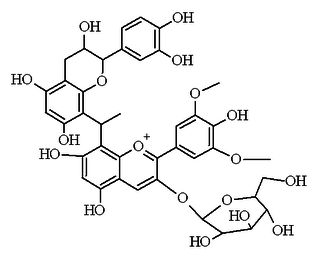
Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.















