Curcuma alismatifolia, Siam tulip or summer tulip is a tropical plant native to Laos, northern Thailand, and Cambodia. Despite its name, it is not related to the tulip, but is a close relative of turmeric. It can grow as an indoor plant. It is a perennial herb growing up to 2 feet tall.

Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant. Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape variety Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates, and bilberries.
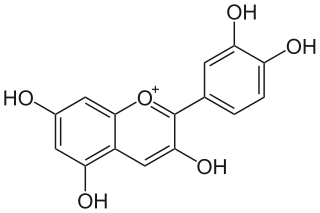
Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.

Malvin is a naturally occurring chemical of the anthocyanin family.

Pelargonidin is an anthocyanidin, a type of plant pigment producing a characteristic orange color used in food and industrial dyes.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart named a chemical compound that gives flowers a blue color, Anthokyan, in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

Phenolic compounds—natural phenol and polyphenols—occur naturally in wine. These include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Petunidin (Pt), like Europinidin and Malvidin, is derived from Delphinidin and is an O-methylated anthocyanidin of the 3-hydroxy type. It is a natural organic compound, a dark-red or purple water-soluble pigment found in many red berries including chokeberries, Saskatoon berries or different species of grape, and also part of the pigments responsible for the petal colors in many flowers. This pigment gives the Indigo Rose tomatoes the majority of their deep purple color when the fruits are exposed to sunlight. The name of the molecule itself is derived from the word Petunia.

Daidzin is a natural organic compound in the class of phytochemicals known as isoflavones. Daidzin can be found in Japanese plant kudzu and from soybean leaves.

Aromadendrin is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.
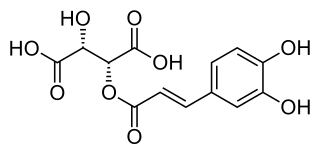
Caftaric acid is a non-flavonoid phenolic compound.

Oenin is an anthocyanin. It is the 3-glucoside of malvidin. It is one of the red pigments found in the skin of purple grapes and in wine.
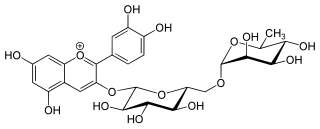
Antirrhinin is an anthocyanin. It is the 3-rutinoside of cyanidin.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

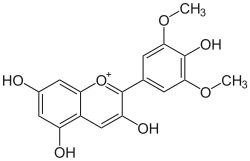
Syringetin is an O-methylated flavonol, a type of flavonoid. It is found in red grape, in Lysimachia congestiflora and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.

Patuletin is an O-methylated flavonol. It can be found in the genus Eriocaulon.

Malvidin glucoside-ethyl-catechin is a flavanol-anthocyanin adduct. Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.

Violdelphin is an anthocyanin, a plant pigment, has been found in the purplish blue flower of Aconitum chinense, in the blue flowers in the genus Campanula and in the blue flowers of Delphinium hybridum. It is a flavenoid natural product, incorporating two p-hydroxy benzoic acid residues, one rutinoside and two glucosides associated with a delphinidin.

Silene jenisseensis, is a species of flowering plant in the family Caryophyllaceae, native to Siberia, Far East and Mongolia.