
The blackcurrant, also known as black currant or cassis, is a deciduous shrub in the family Grossulariaceae grown for its edible berries. It is native to temperate parts of central and northern Europe and northern Asia, where it prefers damp fertile soils. It is widely cultivated both commercially and domestically.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Hydrangea macrophylla is a species of flowering plant in the family Hydrangeaceae, native to Japan. It is a deciduous shrub growing to 2 m (7 ft) tall by 2.5 m (8 ft) broad with large heads of pink or blue flowers in summer and autumn. Common names include bigleaf hydrangea, French hydrangea, lacecap hydrangea, mophead hydrangea, and hortensia. It is widely cultivated in many parts of the world in many climates. It is not to be confused with H. aspera 'Macrophylla'.

Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant. Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape variety Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates, and bilberries.
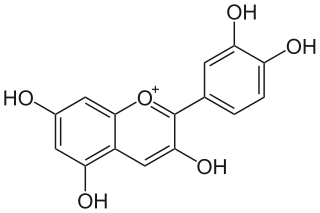
Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

In enzymology, a flavonol 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction

Gentiana triflora is a tall, flowering perennial plant in the genus Gentiana native to higher-elevation meadows and forests of China, Mongolia, Eastern Russia, Korea and Japan.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Petunidin (Pt), like Europinidin and Malvidin, is derived from Delphinidin and is an O-methylated anthocyanidin of the 3-hydroxy type. It is a natural organic compound, a dark-red or purple water-soluble pigment found in many redberries including chokeberries, Saskatoon berries or different species of grape, and also part of the pigments responsible for the petal colors in many flowers. This pigment gives the Indigo Rose tomatoes the majority of their deep purple color when the fruits are exposed to sunlight. The name of the molecule itself is derived from the word Petunia.

Aristotelia chilensis, known as maqui or Chilean wineberry, is a tree species in the Elaeocarpaceae family native to South America in the Valdivian temperate forests of Chile and adjacent regions of southern Argentina. Limited numbers of these trees are cultivated in gardens for their small fruits. Wild-harvested fruits are commercially marketed.

A metalloanthocyanin is a chemical complex giving color to petals of certain plants.

Chrysanthemin is an anthocyanin. It is the 3-glucoside of cyanidin.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and other phenylpropanoids.

Delphinidin 3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin. It can be found in some red Vitis vinifera grape cultivars and in red wine.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).
Anthocyanin 3-O-glucoside 6″-O-hydroxycinnamoyltransferase is an enzyme forming delphinidin 3-(6-p-coumaroyl)glucoside from delphinidin 3-O-glucoside (myrtillin) and p-coumaroyl-CoA.
Delphinidin 3',5'-O-glucosyltransferase is an enzyme with systematic name UDP-glucose:delphinidin 3-O-(6-O-malonyl)-beta-D-glucoside 3'-O-glucosyltransferase. This enzyme catalyses the following chemical reaction

Blue flower colour was always associated with something unusual and desired. Blue roses especially were assumed to be a dream that cannot be realised. Blue colour in flower petals is caused by anthocyanins, which are members of flavonoid class metabolites. We can diversify three main classes of anthocyanin pigments: cyaniding type responsible for red coloration, pelargonidin type responsible for orange colour and delphinidin type responsible for violet/blue flower and fruits coloration. The main difference in the structure of listed anthocyanins type is the number of hydroxyl groups in the B-ring of the anthocyanin. Nevertheless, in the monomeric state anthocyanins never show blue colour in the weak acidic and neutral pH. The mechanism of blue colour formation are very complicated in most cases, presence of delphinidin type pigments is not sufficient, great role play also the pH and the formation of complexes of anthocyanins with flavones and metal ions.
















