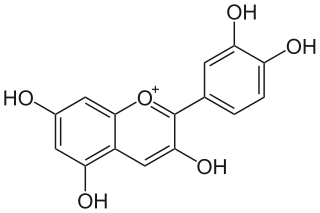
Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.

Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol, a type of flavonoid, found in a variety of plants and plant-derived foods including kale, beans, tea, spinach, and broccoli. Kaempferol is a yellow crystalline solid with a melting point of 276–278 °C (529–532 °F). It is slightly soluble in water and highly soluble in hot ethanol, ethers, and DMSO. Kaempferol is named for 17th-century German naturalist Engelbert Kaempfer.
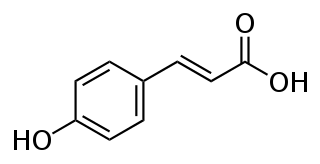
p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.

The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.

Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one).

Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone.
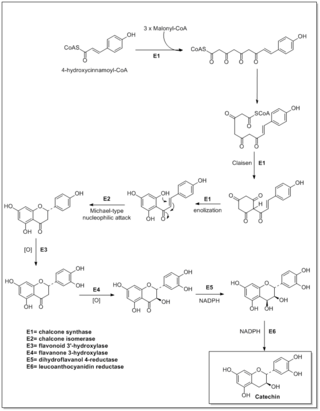
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.
In enzymology, a 4-coumarate—CoA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a flavonol-3-O-triglucoside O-coumaroyltransferase is an enzyme that catalyzes the chemical reaction

Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran and sugarcane.
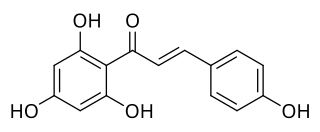
Naringenin chalcone is a common chalconoid. It is synthesized from 4-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS), a key enzyme in the phenylpropanoid pathway. Naringenin chalcone can spontaneously cyclize to naringenin. In plant cells, this process is catalyzed by chalcone isomerase.

Xanthohumol is a natural product found in the female inflorescences of Humulus lupulus, also known as hops. This compound is also found in beer and belongs to a class of compounds that contribute to the bitterness and flavor of hops. Xanthohumol is a prenylated chalconoid, biosynthesized by a type III polyketide synthase (PKS) and subsequent modifying enzymes.
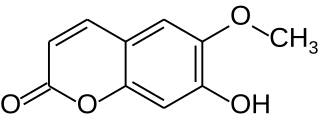
Scopoletin is a coumarin found in the root of plants in the genus Scopolia such as Scopolia carniolica and Scopolia japonica, in chicory, in Artemisia scoparia, in the roots and leaves of stinging nettle, in the passion flower, in Brunfelsia, in Viburnum prunifolium, in Solanum nigrum, in Datura metel, in Mallotus resinosus, or and in Kleinhovia hospita. It can also be found in fenugreek, vinegar, some whiskies or in dandelion coffee. A similar coumarin is scoparone. Scopoletin is highly fluorescent when dissolved in DMSO or water and is regularly used as a fluorimetric assay for the detection of hydrogen peroxide in conjunction with horseradish peroxidase. When oxidized, its fluorescence is strongly suppressed.
The biosynthesis of phenylpropanoids involves a number of enzymes.

Delphinidin 3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin. It can be found in some red Vitis vinifera grape cultivars and in red wine.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).
Anthocyanin 3-O-glucoside 6″-O-hydroxycinnamoyltransferase is an enzyme forming delphinidin 3-(6-p-coumaroyl)glucoside from delphinidin 3-O-glucoside (myrtillin) and p-coumaroyl-CoA.

















