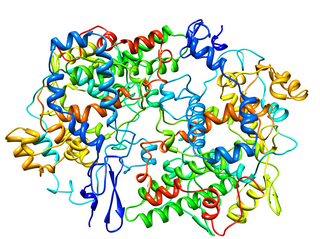
Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for biosynthesis of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.
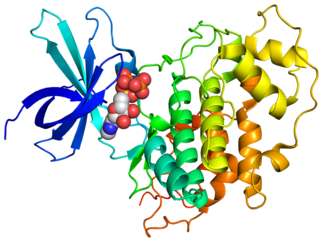
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen synthase (GS), GSK-3 has since been identified as a protein kinase for over 100 different proteins in a variety of different pathways. In mammals, including humans, GSK-3 exists in two isozymes encoded by two homologous genes GSK-3α (GSK3A) and GSK-3β (GSK3B). GSK-3 has been the subject of much research since it has been implicated in a number of diseases, including type 2 diabetes, Alzheimer's disease, inflammation, cancer, addiction and bipolar disorder.

Phellodendron amurense is a species of tree in the family Rutaceae, commonly called the Amur cork tree. It is a major source of huáng bò, one of the 50 fundamental herbs used in traditional Chinese medicine. The Ainu people used this plant, called shikerebe-ni, as a painkiller. It is known as hwangbyeok in Korean and (キハダ) kihada in Japanese.

Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme catalyzes sequential hydroxylations of the steroid angular methyl group at C18 after initial 11β-hydroxylation. It is encoded by the CYP11B2 gene in humans.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.
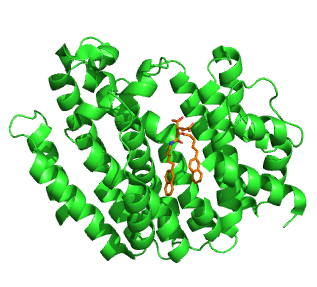
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.

Swietenia macrophylla, commonly known as mahogany, Honduran mahogany, Honduras mahogany, or big-leaf mahogany is a species of plant in the Meliaceae family. It is one of three species that yields genuine mahogany timber (Swietenia), the others being Swietenia mahagoni and Swietenia humilis. It is native to South America, Mexico and Central America, but naturalized in the Philippines, Singapore, Malaysia and Hawaii, and cultivated in plantations and wind-breaks elsewhere.

Mitogen-activated protein kinase kinase kinase 7 (MAP3K7), also known as TAK1, is an enzyme that in humans is encoded by the MAP3K7 gene.

Lapaquistat (TAK-475) is a cholesterol-lowering drug candidate that was abandoned before being marketed.
Ulinastatin, is a glycoprotein that is isolated from healthy human urine or synthetically produced and has molecular weight of 25 - 40kDa. It acts as a urinary trypsin inhibitor (UTI). Highly purified ulinastatin has been clinically used for the treatment of acute pancreatitis, chronic pancreatitis, Stevens–Johnson syndrome, burns, septic shock, and toxic epidermal necrolysis (TEN).

Aspirin causes several different effects in the body, mainly the reduction of inflammation, analgesia, the prevention of clotting, and the reduction of fever. Much of this is believed to be due to decreased production of prostaglandins and TXA2. Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (COX) enzyme. Cyclooxygenase is required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the COX enzyme. This makes aspirin different from other NSAIDs, which are reversible inhibitors; aspirin creates an allosteric change in the structure of the COX enzyme. However, other effects of aspirin, such as uncoupling oxidative phosphorylation in mitochondria, and the modulation of signaling through NF-κB, are also being investigated. Some of its effects are like those of salicylic acid, which is not an acetylating agent.
Inte:Ligand was founded in Maria Enzersdorf, Lower Austria (Niederösterreich) in 2003. They established the company headquarters on Mariahilferstrasse in Vienna, Austria that same year.

Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols. It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers. Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.

Morin is a yellow chemical compound that can be isolated from Maclura pomifera (Osage orange), Maclura tinctoria (old fustic), and from leaves of Psidium guajava (common guava). In a preclinical in vitro study, morin was found to be a weak inhibitor of fatty acid synthase with an IC50 of 2.33 μM. Morin was also found to inhibit amyloid formation by islet amyloid polypeptide (or amylin) and disaggregate amyloid fibers.

Huáng bǎi, huáng bó or huáng bò is one of the fifty fundamental herbs of traditional Chinese medicine. Known also as Cortex Phellodendri, it is the bark of one of two species of Phellodendron tree: Phellodendron amurense or Phellodendron chinense.

Withaferin A is a steroidal lactone, derived from Acnistus arborescens, Withania somnifera and other members of family Solanaceae. It is the first member of the withanolide class of ergostane type product to be discovered.
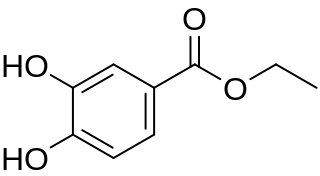
Ethyl protocatechuate is a phenolic compound. It can be found in the peanut seed testa. It is also present in wine. It is the ethylic ester of protocatechuic acid.

(S)-Magnoflorine is a quaternary benzylisoquinoline alkaloid (BIA) of the aporphine structural subgroup which has been isolated from various species of the family Menispermaceae, such as Pachygone ovata,Sinomenium acutum, and Cissampelos pareira.

Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone) is a naturally occurring para-benzoquinone isolated from dried berries of Embelia ribes plants. Embelin has a wide spectrum of biological activities, including antioxidant, antitumor, anti-inflammatory, analgesic, anthelmintic, antifertility and antimicrobial. Several studies have reported antidiabetic activity of embelin Embelin treatment significantly decreased paraquat‐induced lung injury through suppressing oxidative stress, inflammatory cascade, and MAPK/NF‐κB signaling pathway in paraquat‐intoxicated rats Embelin and embelin derivatives selectively inhibits 5-LOX and microsomal prostaglandin E2 synthase-1



















