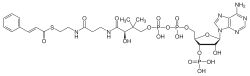 | |
| Names | |
|---|---|
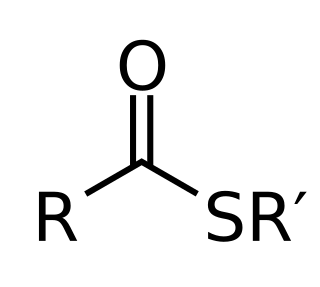
| IUPAC name 3′-O-Phosphonoadenosine 5′-[(3R)-3-hydroxy-2,2-dimethyl-4-({3-[(2-{[(2E)-3-phenylprop-2-enoyl]sulfanyl}ethyl)amino]-3-oxopropyl}amino)-4-oxobutyl dihydroxen diphosphate] | |
| Systematic IUPAC name [(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl (3R)-3-hydroxy-2,2-dimethyl-4-({3-[(2-{[(2E)-3-phenylprop-2-enoyl]sulfanyl}ethyl)amino]-3-oxopropyl}amino)-4-oxobutyl dihydrogen diphosphate | |
| Other names Cinnamoyl-coa (E)-cinnamoyl-CoA Coenzyme A, S-(3-phenyl-2-propenoate) (E)-benzylideneacetyl-CoA 3-phenylacryloyl-CoA | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C30H42N7O17P3S | |
| Molar mass | 897.68 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Cinnamoyl-coenzyme A is an intermediate in the phenylpropanoid metabolic pathway.