
Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.

In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the binding site, and residues that catalyse a reaction of that substrate, the catalytic site. Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes.

Cynara is a genus of thistle-like perennial plants in the family Asteraceae. They are native to the Mediterranean region, the Middle East, northwestern Africa, and the Canary Islands. The genus name comes from the Greek kynara, which means "artichoke".
Project Artichoke was a project developed and enacted by the United States Central Intelligence Agency (CIA) for the purpose of researching methods of interrogation.

Paint stripper or paint remover is a chemical product designed to remove paint, finishes, and coatings, while also cleaning the underlying surface.
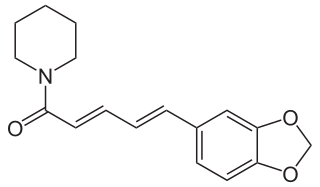
Piperine, possibly along with its isomer chavicine, is the compound responsible for the pungency of black pepper and long pepper. It has been used in some forms of traditional medicine.

Chlorogenic acid (CGA) is the ester of caffeic acid and (−)-quinic acid, functioning as an intermediate in lignin biosynthesis. The term "chlorogenic acids" refers to a related polyphenol family of esters, including hydroxycinnamic acids with quinic acid.

Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of slightly below 1.
Calcium hypochlorite is an inorganic compound with formula Ca(ClO)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.
A chemical depilatory is a cosmetic preparation used to remove hair from the skin. Common active ingredients are salts of thioglycolic acid and thiolactic acids. These compounds break the disulfide bonds in keratin and also hydrolyze the hair so that it is easily removed. Formerly, sulfides such as strontium sulfide were used, but due to their unpleasant odor, they have been replaced by thiols.
Activation, in chemistry and biology, is the process whereby something is prepared or excited for a subsequent reaction.
Hydroxycinnamic acids (hydroxycinnamates) are a class of aromatic acids or phenylpropanoids having a C6–C3 skeleton. These compounds are hydroxy derivatives of cinnamic acid.

Enzyme catalysis is the increase in the rate of a process by a biological molecule, an "enzyme". Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, called the active site.
In enzymology, a D-lactate dehydrogenase (cytochrome) is an enzyme that catalyzes the chemical reaction

Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.
Inulinase is an enzyme with systematic name 1-β-D-fructan fructanohydrolase. It catalyses the reaction

Doisynolic acid is a synthetic, orally active, nonsteroidal estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, whose levorotatory isomer is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.












