
Bread is a staple food prepared from a dough of flour and water, usually by baking. Throughout recorded history, it has been a prominent food in large parts of the world. It is one of the oldest human-made foods, having been of significant importance since the dawn of agriculture, and plays an essential role in both religious rituals and secular culture.

Flax, also known as common flax or linseed, is a flowering plant, Linum usitatissimum, in the family Linaceae. It is cultivated as a food and fiber crop in regions of the world with temperate climate. Textiles made from flax are known in Western countries as linen, and are traditionally used for bed sheets, underclothes, and table linen. Its oil is known as linseed oil. In addition to referring to the plant itself, the word "flax" may refer to the unspun fibers of the flax plant. The plant species is known only as a cultivated plant, and appears to have been domesticated just once from the wild species Linum bienne, called pale flax. The plants called "flax" in New Zealand are, by contrast, members of the genus Phormium.
A glucoside is a glycoside that is derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.
![Secoisolariciresinol diglucoside Antioxidant[1] phytoestrogen present in flax, sunflower, sesame, and pumpkin seeds. In food, it can be found in commercial breads containing flaxseed](https://upload.wikimedia.org/wikipedia/commons/thumb/b/b2/Secoisolarisiresinol_diglucoside.svg/320px-Secoisolarisiresinol_diglucoside.svg.png)
Secoisolariciresinol diglucoside (SDG) is an antioxidant phytoestrogen present in flax, sunflower, sesame, and pumpkin seeds. In food, it can be found in commercial breads containing flaxseed. It is a precursor of mammal lignans which are produced in the colon from chemicals in foods.

Ferulic acid is a hydroxycinnamic acid, an organic compound. It is an abundant phenolic phytochemical found in plant cell walls, covalently bonded as side chains to molecules such as arabinoxylans. As a component of lignin, ferulic acid is a precursor in the manufacture of other aromatic compounds. The name is derived from the genus Ferula, referring to the giant fennel.
Coumaric acid is a phenolic derivative of cinnamic acid having a hydroxy group as substituent at one of the aromatic positions:

p-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acid—o-coumaric acid, m-coumaric acid, and p-coumaric acid—that differ by the position of the hydroxy substitution of the phenyl group. p-Coumaric acid is the most abundant isomer of the three in nature. p-Coumaric acid exists in two forms trans-p-coumaric acid and cis-p-coumaric acid.
4-Ethylphenol (4-EP) is a phenolic compound.

The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.
The molecular formula C15H18O8 (molar mass: 326.29 g/mol, exact mass: 326.100168 u) may refer to:

Phenolic acids or phenolcarboxylic acids are types of aromatic acid compound. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function. Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.
Tergallic acids are trimers of gallic acid, often found naturally in the form of glycosides. Tergallic acid O- or C-glucosides that can be found in acorns of several Quercus (oak) species. The dehydrated tergallic acid C-glucoside and tergallic acid O-glucoside can be characterised in the acorns of Quercus macrocarpa. Dehydrated tergallic-C-glucoside can be found in the cork from Quercus suber.
4-Vinylphenol is a phenolic compound found in wine and beer. It is produced by the spoilage yeast Brettanomyces. When it reaches concentrations greater than the sensory threshold, it can give the wine aromas described as barnyard, medicinal, band-aids, and mousy. In wine, 4-vinylphenol can react with other molecules, such as anthocyanidins, to produce new chemical compounds. In white wines vinylphenols are dominant whereas, in red wines, it is the corresponding ethyl phenols.
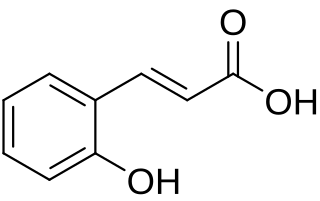
o-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acids — o-coumaric acid, m-coumaric acid, and p-coumaric acid — that differ by the position of the hydroxy substitution of the phenyl group.

m-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acid – o-coumaric acid, m-coumaric acid, and p-coumaric acid – that differ by the position of the hydroxy substitution of the phenyl group.

Malvidin-3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin found in grape and wine. There are two forms with the cis and trans isomers of p-coumaric acid. It is a cation.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).

4-Hydroxybenzoic acid 4-O-glucoside is a glucoside of p-hydroxybenzoic acid. It can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces.


![Secoisolariciresinol diglucoside Antioxidant[1] phytoestrogen present in flax, sunflower, sesame, and pumpkin seeds. In food, it can be found in commercial breads containing flaxseed](https://upload.wikimedia.org/wikipedia/commons/thumb/b/b2/Secoisolarisiresinol_diglucoside.svg/320px-Secoisolarisiresinol_diglucoside.svg.png)









