
Echinacea is a genus of herbaceous flowering plants in the daisy family. It has ten species, which are commonly called coneflowers. They are found only in eastern and central North America, where they grow in moist to dry prairies and open wooded areas. They have large, showy heads of composite flowers, blooming in summer. The generic name is derived from the Greek word ἐχῖνος, meaning "hedgehog", due to the spiny central disk. These flowering plants and their parts have different uses. Some species are cultivated in gardens for their showy flowers. Two of the species, E. tennesseensis and E. laevigata, were formerly listed in the United States as endangered species; E. tennesseensis has been delisted due to recovery and E. laevigata is now listed as threatened.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
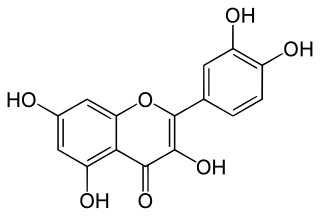
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.

Echinacea angustifolia, the narrow-leaved purple coneflower or blacksamson echinacea, is a species of flowering plant in the family Asteraceae. It is native to North America, where it is widespread across much of the Great Plains of central Canada and the central United States, with additional populations in surrounding regions.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.
Rhamnose is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L-arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as Pseudomonas aeruginosa and Helicobacter pylori.

Ipomoea purpurea, the common morning-glory, tall morning-glory, or purple morning glory, is a species in the genus Ipomoea, native to Mexico and Central America.
Hydroxycinnamic acids (hydroxycinnamates) are a class of aromatic acids or phenylpropanoids having a C6–C3 skeleton. These compounds are hydroxy derivatives of cinnamic acid.

Echinacea purpurea, the eastern purple coneflower, purple coneflower, hedgehog coneflower, or echinacea, is a North American species of flowering plant in the family Asteraceae. It is native to parts of eastern North America and present to some extent in the wild in much of the eastern, southeastern and midwestern United States as well as in the Canadian Province of Ontario. It is most common in the Ozarks and in the Mississippi/Ohio Valley. Its habitats include dry open woods, prairies and barrens.

Galinsoga parviflora is a herbaceous plant in the Asteraceae (daisy) family. It has several common names including guasca (Colombia), pacpa yuyo, paco yuyo, and waskha (Peru), burrionera (Ecuador), albahaca silvestre and saetilla (Argentina), mielcilla, piojito, galinsoga, gallant soldier, quickweed, and potato weed.

Echinacea pallida, the pale purple coneflower, is a species of herbaceous perennial plant in the family Asteraceae. It is sometimes grown in gardens and used for medicinal purposes. Its native range is the central region of the United States and Ontario, Canada.

Dandelion 'coffee' is a tisane made from the root of the dandelion plant. The roasted dandelion root pieces and the beverage have some resemblance to coffee in appearance and taste, and it is thus commonly considered a coffee substitute. Dandelion root is used for both medicinal and culinary purposes and is thought to be a detoxifying herb.

Chicoric acid is a hydroxycinnamic acid, an organic compound of the phenylpropanoid class and occurs in a variety of plant species. It is a derivative of both caffeic acid and tartaric acid.
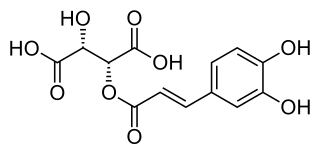
Caftaric acid is a non-flavonoid phenolic compound.

Eupatolitin is a chemical compound. It is an O-methylated flavonol, a type of flavonoid. Eupatolitin can be found in Brickellia veronicaefolia and in Ipomopsis aggregata.

Patuletin is an O-methylated flavonol. It can be found in the genus Eriocaulon.

Phenolic acids or phenolcarboxylic acids are types of aromatic acid compounds. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function. Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.

Verbascoside is a caffeoyl phenylethanoid glycoside in which the phenylpropanoid caffeic acid and the phenylethanoid hydroxytyrosol form an ester and an ether bond respectively, to the rhamnose part of a disaccharide, namely β-(3′,4′-dihydroxyphenyl)ethyl-O-α-L-rhamnopyranosyl(1→3)-β-D-(4-O-caffeoyl)-glucopyranoside.

Homoisoflavonoids (3-benzylidenechroman-4-ones) are a type of phenolic compounds occurring naturally in plants.

















