
p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.

Malvidin is an O-methylated anthocyanidin, the 3',5'-methoxy derivative of delphinidin. As a primary plant pigment, its glycosides are highly abundant in nature.

Betalains are a class of red and yellow tyrosine-derived pigments found in plants of the order Caryophyllales, where they replace anthocyanin pigments. Betalains also occur in some higher order fungi. They are most often noticeable in the petals of flowers, but may color the fruits, leaves, stems, and roots of plants that contain them. They include pigments such as those found in beets.

Malva sylvestris is a species of the mallow genus Malva in the family of Malvaceae and is considered to be the type species for the genus. Known as common mallow to English-speaking Europeans, it acquired the common names of cheeses, high mallow and tall mallow as it migrated from its native home in Western Europe, North Africa and Asia through the English-speaking world.
In enzymology, a flavanone 7-O-beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a sterol 3beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise “Die Farben der Blüthen”. Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

The color of wine is one of the most easily recognizable characteristics of wines. Color is also an element in wine tasting since heavy wines generally have a deeper color. The accessory traditionally used to judge the wine color was the tastevin, a shallow cup allowing one to see the color of the liquid in the dim light of a cellar. The color is an element in the classification of wines.
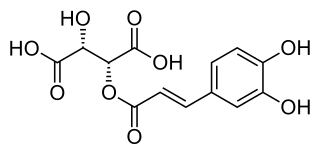
Caftaric acid is a non-flavonoid phenolic compound.

Oenin is an anthocyanin. It is the 3-glucoside of malvidin. It is one of the red pigments found in the skin of purple grapes and in wine.
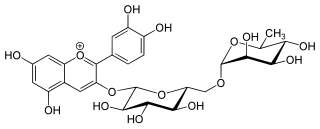
Antirrhinin is an anthocyanin. It is the 3-rutinoside of cyanidin.
Sambubiose is a disaccharide. It is the β-D-xylosyl-(1→2)-β-D-glucose.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

Syringetin is an O-methylated flavonol, a type of flavonoid. It is found in red grape, in Lysimachia congestiflora and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.
Copigmentation is a phenomenon where pigmentation due to anthocyanidins is reinforced by the presence of other colorless flavonoids known as cofactors or “copigments”. This occurs by the formation of a non-covalently-linked complex.
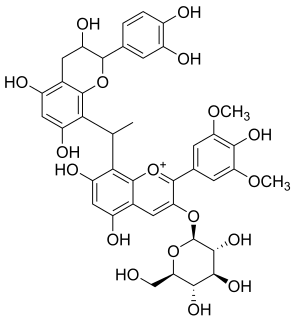
Malvidin glucoside-ethyl-catechin is a flavanol-anthocyanin adduct. Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.
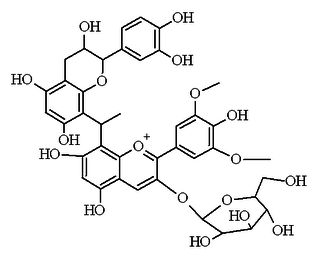
Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.

Violdelphin is an anthocyanin, a plant pigment, has been found in the purplish blue flower of Aconitum chinense, in the blue flowers in the genus Campanula and in the blue flowers of Delphinium hybridum. It is a flavenoid natural product, incorporating two p-hydroxy benzoic acid residues, one rutinoside and two glucosides associated with a delphinidin.
Anthocyanin 5-O-glucosyltransferase is an enzyme that forms anthocyanin 3,5-O-diglucoside from anthocyanin 3-O-glucoside.














