Related Research Articles
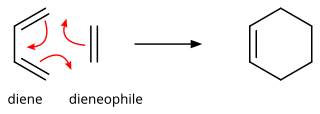
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels–Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.

In organic chemistry, the benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals, and results in formation of an acyloin. In the classic example, benzaldehyde is converted to benzoin.
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.

Carl Dietrich Harries was a German chemist born in Luckenwalde, Brandenburg, Prussia. He received his doctorate in 1892. In 1900, he married Hertha von Siemens, daughter of the electrical genius Werner von Siemens, and the inventor of one of the earliest ozone generators. In 1904, he moved as full professor to the University of Kiel, where he remained until 1916. During that time he published numerous papers on ozonolysis. His major publication detailing ozonolysis was published in Liebigs Ann. Chem. 1905, 343, 311. Dissatisfied with academic life and having failed to obtain either of two positions at universities, he left academia to become director of research at Siemens and Halske. He died on 3 November 1923 of complications following surgery for cancer. He was buried at Stahnsdorf South-Western Cemetery near Berlin.

Wilhelm Rudolph Fittig was a German chemist. He discovered the pinacol coupling reaction, mesitylene, diacetyl and biphenyl. Fittig studied the action of sodium on ketones and hydrocarbons. He discovered the Fittig reaction or Wurtz–Fittig reaction for the synthesis of alkylbenzenes, he proposed a diketone structure for benzoquinone and isolated phenanthrene from coal tar. He discovered and synthesized the first lactones and investigated structures of piperine, naphthalene, and fluorene.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds. Note that trihalomethyl ketone substrates will result in haloform and carboxylate formation via the haloform reaction instead.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed. The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.

The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908.

Nikolay Nikolaevich Zinin was a Russian organic chemist.
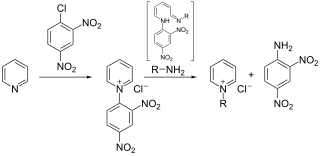
The Zincke reaction is an organic reaction, named after Theodor Zincke, in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine.

Zincke aldehydes, or 5-aminopenta-2,4-dienals, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines the Zincke reaction takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal iminium group hydrolyzed to an aldehyde. The use of the dinitrophenyl group for pyridine activation was first reported by Theodor Zincke. The use of cyanogen bromide for pyridine activation was independently reported by W. König:

Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole.

Heinrich Limpricht was a German chemist. Limpricht was a pupil of Friedrich Wöhler; he worked on the chemistry of furans and pyrroles, discovering furan in 1870.

Otto Dimroth was a German chemist. He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.

Alkannin is a natural dye that is obtained from the extracts of the plant dyer's alkanet (Alkanna tinctoria) which is found in the Mediterranean region. The dye is used as a food coloring and in cosmetics; within the European E number schedule, it is numbered E103. It is used as a red-brown food additive in regions such as Australia. Alkannin is deep red in an acid and blue in an alkaline environment. The chemical structure as a naphthoquinone derivative was first determined by Hans Brockmann in 1936. The (R)-enantiomer of alkannin is known as shikonin, and the racemic mixture of the two is known as shikalkin.
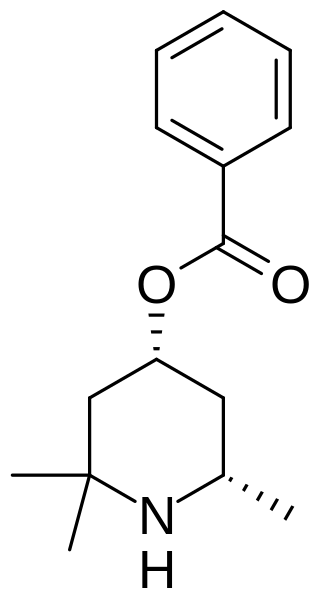
Eucaine, also known as β-eucaine or Betacaine, is a drug that was previously used as a local anesthetic. It was designed as an analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic. It is a white, crystalline solid. Prior to World War I, Britain imported eucaine from Germany. During the war, a team including Jocelyn Field Thorpe and Martha Annie Whiteley developed a synthesis in Britain.

Blue flowers are rare in nature, and despite many attempts, blue roses, carnations and chrysanthemums in particular cannot not be produced by conventional breeding techniques. Blue colour in flower petals is caused by delphinidin, a type of anthocyanin, which are a class of flavonoids.
References
- ↑ Willstätter R & Everest RW (1913). "Untersuchungen über die Anthocyane. I. Über den Farbstoff der Kornblume". Justus Liebigs Ann. Chem. 401 (2): 189–232. doi:10.1002/jlac.19134010205.
- 1 2 Willstätter R, Mallison H (1915). "Untersuchungen über die Anthocyane. X. Über Variationen der Blütenfarben". Justus Liebigs Ann. Chem. 408: 147–162. doi:10.1002/jlac.19154080110.
- ↑ Shiono M, Matsugaki N, Takeda K (2005). "Structure of the blue cornflower pigment". Nature. 436 (7052): 791. Bibcode:2005Natur.436..791S. doi: 10.1038/436791a . PMID 16094358. S2CID 4312804.