
Vitis vinifera, the common grape vine, is a species of Vitis, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Catechin is a flavan-3-ol, a type of natural phenol and antioxidant. It is a plant secondary metabolite. It belongs to the group of flavan-3-ols, part of the chemical family of flavonoids.

Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant. Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape that produces Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates, and bilberries.
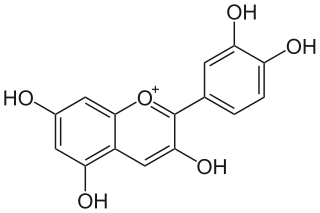
Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Malvin is a naturally occurring chemical of the anthocyanin family.

Malvidin is an O-methylated anthocyanidin, the 3',5'-methoxy derivative of delphinidin. As a primary plant pigment, its glycosides are highly abundant in nature.

Anthocyanins are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue or black. Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

The color of wine is one of the most easily recognizable characteristics of wines. Color is also an element in wine tasting since heavy wines generally have a deeper color. The accessory traditionally used to judge the wine color was the tastevin, a shallow cup allowing one to see the color of the liquid in the dim light of a cellar. The color is an element in the classification of wines.
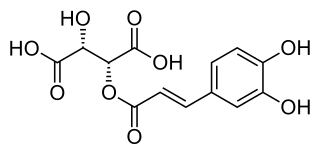
Caftaric acid is a non-flavonoid phenolic compound.

Oenin is an anthocyanin. It is the 3-glucoside of malvidin. It is one of the red pigments found in the skin of purple grapes and in wine.

Myrtillin is an anthocyanin. It is the 3-glucoside of delphinidin. It can be found in all green plants, most abundantly in blackcurrant, blueberry, huckleberry, bilberry leaves and in various myrtles, roselle plants, and Centella asiatica plant. It is also present in yeast and oatmeal. The sumac fruit's pericarp owes its dark red colour to anthocyanin pigments, of which chrysanthemin, myrtillin and delphinidin have yet been identified.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
Copigmentation is a phenomenon where pigmentation due to anthocyanidins is reinforced by the presence of other colorless flavonoids known as cofactors or “copigments”. This occurs by the formation of a non-covalently-linked complex.

Vitisin A is a natural phenol found in red wines. It is a pyranoanthocyanin.

Vitisin B is a natural phenol found in red wines. It is a pyranoanthocyanin.
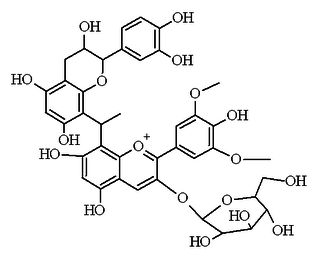
Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.

Oxovitisins are a type of pyranoanthocyanin with a pyranone (2-pyrone) component found in aged Port wines. They do not contain an oxonium ion component, as anthocyanins do. Therefore, they do not have an absorption maximum at 520 nm. Oxovitisins are stable yellowish pigments with similar unique spectral features, displaying only a pronounced broad band around 370 nm in the UV−vis spectrum.

Malvidin-3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin found in grape and wine. There are two forms with the cis and trans isomers of p-coumaric acid. It is a cation.


















