Tannins are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They are found in most plants and have a role in plant defense.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Schinopsis balansae is a hardwood tree known as willow-leaf red quebracho which forms forests in the subtropical Humid Chaco ecoregion of north-eastern Argentina, and Paraguay. It is also found in the wild Pantanal vegetation in Brazil. Some of its vernacular names are quebracho colorado chaqueño and quebracho santafesino. Other species, like Schinopsis lorentzii, bear the general name quebracho and have similar properties and uses. S. balansae shares its habitat with a species of the same genus, S. heterophylla, and the two are often confused.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Acacia mearnsii, commonly known as black wattle, late black wattle or green wattle, is a species of flowering plant in the family Fabaceae and is endemic to south-eastern Australia. It is usually an erect tree with smooth bark, bipinnate leaves and spherical heads of fragrant pale yellow or cream-coloured flowers followed by black to reddish brown pods. In some other parts of the world, it is regarded as an invasive species.

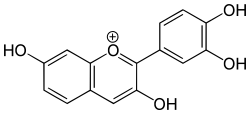
Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

Rhizophora apiculata belongs to the Plantae kingdom under the Rhizophoraceae family. Currently R. apiculata is distributed throughout Australia, Guam, India, Indonesia, Malaysia, Micronesia, New Caledonia, Papua New Guinea, the Philippines, Singapore, the Solomon Islands, Sri Lanka, Taiwan, the Maldives, Thailand, Vanuatu, and Vietnam. Rhizophora apiculata is called ‘bakhaw lalaki,’ in the Philippines, "Thakafathi ތަކަފަތި" in the Maldives, 'Đước' in Vietnam, Garjan in India, as well as other vernacular names.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols. It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers. Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.
A profisetinidin is a type of condensed tannins formed from leuco-fisetinidin, the leucoanthocyanidin form of fisetinidin.
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.
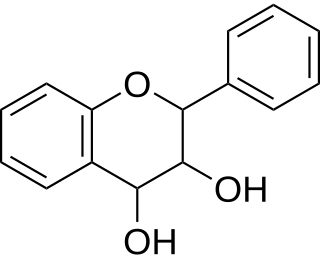
Leucoanthocyanidin (flavan-3,4-diols) are colorless chemical compounds related to anthocyanidins and anthocyanins. Leucoanthocyanins can be found in Anadenanthera peregrina and in several species of Nepenthes including N. burbidgeae, N. muluensis, N. rajah, N. tentaculata, and N. × alisaputrana.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.

Phlobaphenes are reddish, alcohol-soluble and water-insoluble phenolic substances. They can be extracted from plants, or be the result from treatment of tannin extracts with mineral acids. The name phlobaphen come from the Greek roots φλoιὀς (phloios) meaning bark and βαφή (baphe) meaning dye.
Prorobinetidins are a type of condensed tannins formed from robinetinidol. They form robinetinidin when depolymerized under oxidative conditions.

Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.
Quebracho[keˈβ̞ɾa.t͡ʃo] is a common name in Spanish to describe very hard wood tree species. The etymology of the name derived from quiebrahacha, or quebrar hacha, meaning "axe-breaker".

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

Leucofisetinidin is a flavan-3,4-diol (leucoanthocyanidin), a type of natural phenolic substance. It is the monomer of condensed tannins called profisetinidins. Those tannins can be extracted from the heartwood of Acacia mearnsii or from the heartwoods of Schinopsis balansae, Schinopsis quebrachocolorado and from commercial quebracho extract.