
Anthocyanidins are common plant pigments, the sugar-free counterparts of anthocyanins. They are based on the flavylium cation, an oxonium ion, with various groups substituted for its hydrogen atoms. They generally change color from red through purple, blue, and bluish green as a function of pH.

Catharanthus is a genus of flowering plants in the family Apocynaceae. Like the genus Vinca, they are known commonly as periwinkles. There are eight known species. Seven are endemic to Madagascar, though one, C. roseus, is widely naturalized around the world. The eighth species, C. pusillus, is native to India and Sri Lanka. The name Catharanthus comes from the Greek for "pure flower".
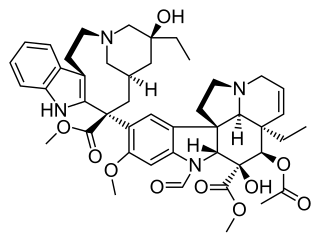
Vincristine, also known as leurocristine and marketed under the brand name Oncovin among others, is a chemotherapy medication used to treat a number of types of cancer. This includes acute lymphocytic leukemia, acute myeloid leukemia, Hodgkin's disease, neuroblastoma, and small cell lung cancer among others. It is given intravenously.

Catharanthus roseus, commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, rose periwinkle, is a perennial species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar, but grown elsewhere as an ornamental and medicinal plant. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus Vinca as Vinca rosea.

Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.

Peonidin is an O-methylated anthocyanidin derived from Cyanidin, and a primary plant pigment. Peonidin gives purplish-red hues to flowers such as the peony, from which it takes its name, and roses. It is also present in some blue flowers, such as the morning glory.
In enzymology, a secologanin synthase (EC 1.14.19.62, was wrongly classified as EC 1.3.3.9 in the past) is an enzyme that catalyzes the chemical reaction
In enzymology, a desacetoxyvindoline 4-hydroxylase (EC 1.14.11.20) is an enzyme that catalyzes the chemical reaction
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

Europinidin (Eu) is an O-methylated anthocyanidin. It is a water-soluble, bluish red plant dye. It is a rare O-methylated flavonoid, a derivative of delphinidin. It can be found in some species of Plumbago and Ceratostigma.

Capensinidin (Cp) is an O-methylated anthocyanidin. It is a water-soluble, blue-red plant dye. It is a 5-methoxy analog of malvidin, has been obtained from Plumbago capensis.

Hirsutidin is an O-methylated anthocyanidin, a chemical compound belonging to the anthocyanins. It can be found in Catharanthus roseus where it is the prominent compound in petals and can also be found in callus cultures.
The O-methylated flavonoids or methoxyflavonoids are flavonoids with methylations on hydroxyl groups. O-methylation has an effect on the solubility of flavonoids.

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Serpentine is a terpene indole alkaloid produced by several members of the family Apocynaceae, including Catharanthus roseus and Rauvolfia serpentina.

Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids. Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine.

16-Hydroxytabersonine is a terpene indole alkaloid produced by the plant Catharanthus roseus. The metabolite is an intermediate in the formation of vindoline, a precursor needed for formation of the pharmaceutically valuable vinblastine and vincristine. 16-hydroxytabersonine is formed from the hydroxylation of tabersonine by tabersonine 16-hydroxylase (T16H). Tabersonine 16-O-methyltransferase (16OMT) methylates the hydroxylated 16 position to form 16-methoxytabersonine.
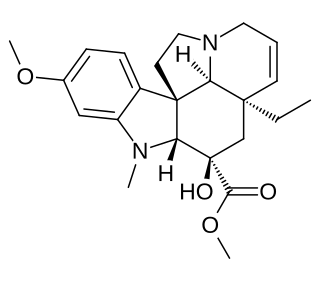
Desacetoxyvindoline is a terpene indole alkaloid produced by the plant Catharanthus roseus. Desacetoxyvindoline is a product formed by the methylation of the nitrogen on the indole ring by the enzyme 3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase (NMT). The metabolite is a substrate for desacetoxyvindoline 4-hydroxylase (D4H) which catalyzes a hydroxylation to yield deacetylvindoline.

Deacetylvindoline is a terpene indole alkaloid produced by Catharanthus roseus. Deacetylvindoline is the product of a hydroxylation of desacetoxyvindoline by deacetoxyvindoline 4-hydroxylase (D4H). It is a substrate for deacetylvindoline O-acetyltransferase (DAT) which acetylates a hydroxy group to form vindoline, one of the two immediate precursors for the formation of the pharmacetucially valuable bisindole alkaloid vinblastine.















