Vaccinium is a common and widespread genus of shrubs or dwarf shrubs in the heath family (Ericaceae). The fruits of many species are eaten by humans and some are of commercial importance, including the cranberry, blueberry, bilberry (whortleberry), lingonberry (cowberry), and huckleberry. Like many other ericaceous plants, they are generally restricted to acidic soils.

Vaccinium vitis-idaea, the lingonberry, partridgeberry, mountain cranberry or cowberry, is a small evergreen shrub in the heath family Ericaceae, that bears edible fruit. It is native to boreal forest and Arctic tundra throughout the Northern Hemisphere, from Europe and Asia to North America. Lingonberries are picked in the wild and used to accompany a variety of dishes in Northern Baltoscandia, Russia, Canada and Alaska. Commercial cultivation is undertaken in the U.S. Pacific Northwest and in many other regions of the world.

Bilberries, or sometimes European blueberries, are a primarily Eurasian species of low-growing shrubs in the genus Vaccinium, bearing edible, dark blue berries. The species most often referred to is Vaccinium myrtillus L., but there are several other closely related species.

Vaccinium macrocarpon is a North American species of cranberry of the subgenus Oxycoccus and genus Vaccinium.

Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant. Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape that produces Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates, and bilberries.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

A polyphenol antioxidant is a hypothetical type of antioxidant containing a polyphenolic substructure and studied in vitro. Numbering over 4,000 distinct species mostly from plants, polyphenols may have antioxidant activity in vitro, but are unlikely to be antioxidants in vivo. Hypothetically, they may affect cell-to-cell signaling, receptor sensitivity, inflammatory enzyme activity or gene regulation, although high-quality clinical research has not confirmed any of these possible effects in humans as of 2020.

Peonidin is an O-methylated anthocyanidin derived from Cyanidin, and a primary plant pigment. Peonidin gives purplish-red hues to flowers such as the peony, from which it takes its name, and roses. It is also present in some blue flowers, such as the morning glory.

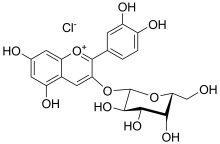
Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Huckleberry is a name used in North America for several plants in the family Ericaceae, in two closely related genera: Vaccinium and Gaylussacia. The huckleberry is the state fruit of Idaho.

Chrysanthemin is an anthocyanin. It is the 3-glucoside of cyanidin.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

Syringetin is an O-methylated flavonol, a type of flavonoid. It is found in red grape, in Lysimachia congestiflora and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.

Laricitrin is an O-methylated flavonol, a type of flavonoid. It is found in red grape and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.
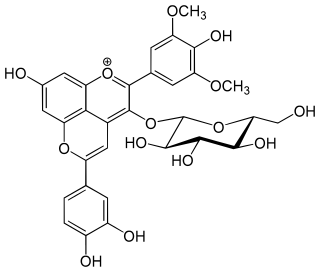
Pinotins are a type of pyranoanthocyanins, a class of phenolic compounds found in red wine. One such compound is pinotin A.

Pinotin A is a pinotin, a type of pyranoanthocyanins and a class of phenolic compounds found in red wine.

Quintinia serrata, the tawheowheo, is a species of evergreen trees in the genus Quintinia endemic to New Zealand.