A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis. Grapes are a non-climacteric type of fruit, generally occurring in clusters.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant. Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape variety Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates, and bilberries.

In enzymology, a flavonol 3-O-glucosyltransferase is an enzyme that catalyzes the chemical reaction

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Petunidin (Pt), like Europinidin and Malvidin, is derived from Delphinidin and is an O-methylated anthocyanidin of the 3-hydroxy type. It is a natural organic compound, a dark-red or purple water-soluble pigment found in many redberries including chokeberries, Saskatoon berries or different species of grape, and also part of the pigments responsible for the petal colors in many flowers. This pigment gives the Indigo Rose tomatoes the majority of their deep purple color when the fruits are exposed to sunlight. The name of the molecule itself is derived from the word Petunia.

The color of wine is one of the most easily recognizable characteristics of wines. Color is also an element in wine tasting since heavy wines generally have a deeper color. The accessory traditionally used to judge the wine color was the tastevin, a shallow cup allowing one to see the color of the liquid in the dim light of a cellar. The color is an element in the classification of wines.
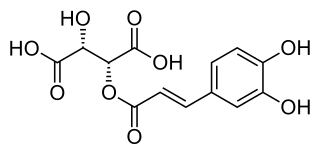
Caftaric acid is a non-flavonoid phenolic compound.

Myrtillin is an anthocyanin. It is the 3-glucoside of delphinidin. It can be found in all green plants, most abundantly in blackcurrant, blueberry, huckleberry, bilberry leaves and in various myrtles, roselle plants, and Centella asiatica plant. It is also present in yeast and oatmeal. The sumac fruit's pericarp owes its dark red colour to anthocyanin pigments, of which chrysanthemin, myrtillin and delphinidin have yet been identified.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

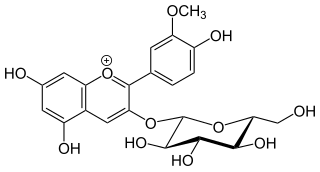
Laricitrin is an O-methylated flavonol, a type of flavonoid. It is found in red grape and in Vaccinium uliginosum. It is one of the phenolic compounds present in wine.

Vitisin A is a natural phenol found in red wines. It is a pyranoanthocyanin.

Petunidin-3-O-glucoside is anthocyanin. It is found in fruits and berries, in red Vitis vinifera grapes and red wine.
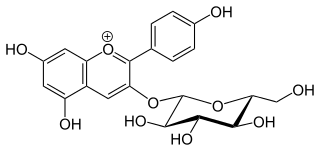
Peonidin-3-O-glucoside is anthocyanin. It is found in fruits and berries, in red Vitis vinifera grapes and red wine, in red onions and in purple corn. It is dark red to purple in colour.

Malvidin glucoside-ethyl-catechin is a flavanol-anthocyanin adduct. Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.

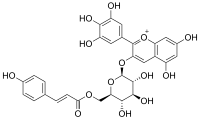
Malvidin-3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin found in grape and wine. There are two forms with the cis and trans isomers of p-coumaric acid. It is a cation.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).
Anthocyanin 3-O-glucoside 6″-O-hydroxycinnamoyltransferase is an enzyme forming delphinidin 3-(6-p-coumaroyl)glucoside from delphinidin 3-O-glucoside (myrtillin) and p-coumaroyl-CoA.
Anthocyanin 5-O-glucosyltransferase is an enzyme that forms anthocyanin 3,5-O-diglucoside from anthocyanin 3-O-glucoside.
Callistephin is an anthocyanin. It is the 3-O-glucoside of pelargonidin.