
The aorta is the main and largest artery in the human body, originating from the left ventricle of the heart, branching upwards immediately after, and extending down to the abdomen, where it splits at the aortic bifurcation into two smaller arteries. The aorta distributes oxygenated blood to all parts of the body through the systemic circulation.
In medicine, a pulse represents the tactile arterial palpation of the cardiac cycle (heartbeat) by fingertips. The pulse may be palpated in any place that allows an artery to be compressed near the surface of the body, such as at the neck, wrist, at the groin, behind the knee, near the ankle joint, and on foot. The radial pulse is commonly measured using three fingers. This has a reason: the finger closest to the heart is used to occlude the pulse pressure, the middle finger is used get a crude estimate of the blood pressure, and the finger most distal to the heart is used to nullify the effect of the ulnar pulse as the two arteries are connected via the palmar arches. The study of the pulse is known as sphygmology.

A heart valve is a biological one-way valve that allows blood to flow in one direction through the chambers of the heart. Four valves are usually present in a mammalian heart and together they determine the pathway of blood flow through the heart. A heart valve opens or closes according to differential blood pressure on each side.

In cardiac physiology, cardiac output (CO), also known as heart output and often denoted by the symbols , , or , is the volumetric flow rate of the heart's pumping output: that is, the volume of blood being pumped by a single ventricle of the heart, per unit time. Cardiac output (CO) is the product of the heart rate (HR), i.e. the number of heartbeats per minute (bpm), and the stroke volume (SV), which is the volume of blood pumped from the left ventricle per beat; thus giving the formula:

Systole is the part of the cardiac cycle during which some chambers of the heart contract after refilling with blood.
Hemodynamics or haemodynamics are the dynamics of blood flow. The circulatory system is controlled by homeostatic mechanisms of autoregulation, just as hydraulic circuits are controlled by control systems. The hemodynamic response continuously monitors and adjusts to conditions in the body and its environment. Hemodynamics explains the physical laws that govern the flow of blood in the blood vessels.
Hemorheology, also spelled haemorheology, or blood rheology, is the study of flow properties of blood and its elements of plasma and cells. Proper tissue perfusion can occur only when blood's rheological properties are within certain levels. Alterations of these properties play significant roles in disease processes. Blood viscosity is determined by plasma viscosity, hematocrit and mechanical properties of red blood cells. Red blood cells have unique mechanical behavior, which can be discussed under the terms erythrocyte deformability and erythrocyte aggregation. Because of that, blood behaves as a non-Newtonian fluid. As such, the viscosity of blood varies with shear rate. Blood becomes less viscous at high shear rates like those experienced with increased flow such as during exercise or in peak-systole. Therefore, blood is a shear-thinning fluid. Contrarily, blood viscosity increases when shear rate goes down with increased vessel diameters or with low flow, such as downstream from an obstruction or in diastole. Blood viscosity also increases with increases in red cell aggregability.

Diastole is the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood. The contrasting phase is systole when the heart chambers are contracting. Atrial diastole is the relaxing of the atria, and ventricular diastole the relaxing of the ventricles.
Vascular resistance is the resistance that must be overcome to push blood through the circulatory system and create blood flow. The resistance offered by the systemic circulation is known as the systemic vascular resistance (SVR) or may sometimes be called by the older term total peripheral resistance (TPR), while the resistance offered by the pulmonary circulation is known as the pulmonary vascular resistance (PVR). Systemic vascular resistance is used in calculations of blood pressure, blood flow, and cardiac function. Vasoconstriction increases SVR, whereas vasodilation decreases SVR.
Compliance is the ability of a hollow organ (vessel) to distend and increase volume with increasing transmural pressure or the tendency of a hollow organ to resist recoil toward its original dimensions on application of a distending or compressing force. It is the reciprocal of "elastance", hence elastance is a measure of the tendency of a hollow organ to recoil toward its original dimensions upon removal of a distending or compressing force.

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.
Pulsus bisferiens, also known as biphasic pulse, is an aortic waveform with two peaks per cardiac cycle, a small one followed by a strong and broad one. It is a sign of problems with the aorta, including aortic stenosis and aortic regurgitation, as well as hypertrophic cardiomyopathy causing subaortic stenosis.
Arterial stiffness occurs as a consequence of biological aging and arteriosclerosis. Inflammation plays a major role in arteriosclerosis development, and consequently it is a major contributor in large arteries stiffening. Increased arterial stiffness is associated with an increased risk of cardiovascular events such as myocardial infarction, hypertension, heart failure and stroke, the two leading causes of death in the developed world. The World Health Organization predicts that in 2010, cardiovascular disease will also be the leading killer in the developing world and represents a major global health problem.
Pulse wave velocity (PWV) is the velocity at which the blood pressure pulse propagates through the circulatory system, usually an artery or a combined length of arteries. PWV is used clinically as a measure of arterial stiffness and can be readily measured non-invasively in humans, with measurement of carotid to femoral PWV (cfPWV) being the recommended method. cfPWV is highly reproducible, and predicts future cardiovascular events and all-cause mortality independent of conventional cardiovascular risk factors. It has been recognized by the European Society of Hypertension as an indicator of target organ damage and a useful additional test in the investigation of hypertension.
A plot of a system's pressure versus volume has long been used to measure the work done by the system and its efficiency. This analysis can be applied to heat engines and pumps, including the heart. A considerable amount of information on cardiac performance can be determined from the pressure vs. volume plot. A number of methods have been determined for measuring PV-loop values experimentally.

The arterial resistivity index, developed by Léandre Pourcelot, is a measure of pulsatile blood flow that reflects the resistance to blood flow caused by microvascular bed distal to the site of measurement.
Continuous noninvasive arterial pressure (CNAP) is the method of measuring beat-to-beat arterial blood pressure in real-time without any interruptions (continuously) and without cannulating the human body (noninvasive).

The hemodynamics of the aorta is an ongoing field of research in which the goal is to identify what flow patterns and subsequent forces occur within the thoracic aorta. These patterns and forces are used to identify the presence and severity of cardiovascular diseases such as aortic aneurysm and atherosclerosis. Some of the methods used to study the hemodynamics of aortic flow are patient scans, computational fluid dynamics models, and particle tracking velocimetry (PTV). The information gathered through these studies can be used for surgery planning and the development of implants. Greater understanding of this topic reduces mortality rates associated with cardiovascular disease.
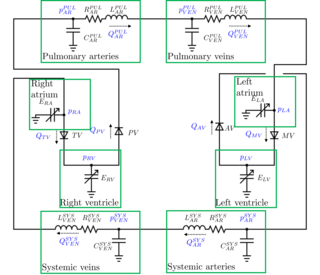
A lumped parameter cardiovascular model is a zero-dimensional mathematical model used to describe the hemodynamics of the cardiovascular system. Given a set of parameters that have a physical meaning, it allows to study the changes in blood pressures or flow rates throughout the cardiovascular system. Modifying the parameters, it is possible to study the effects of a specific disease. For example, arterial hypertension is modeled increasing the arterial resistances of the model.

Wave intensity analysis provides a method to calculate the properties of arterial waves that give rise to arterial blood pressure, based on measurements of pressure, P, and velocity, U, waveforms. Wave intensity analysis is applicable to the evaluation of circulatory physiology and quantifying the pathophysiology of disorders such as coronary artery disease.




















