Heart murmurs are unique heart sounds produced when blood flows across a heart valve or blood vessel. This occurs when turbulent blood flow creates a sound loud enough to hear with a stethoscope. The sound differs from normal heart sounds by their characteristics. For example, heart murmurs may have a distinct pitch, duration and timing. The major way health care providers examine the heart on physical exam is heart auscultation; another clinical technique is palpation, which can detect by touch when such turbulence causes the vibrations called cardiac thrill. A murmur is a sign found during the cardiac exam. Murmurs are of various types and are important in the detection of cardiac and valvular pathologies.

In cardiac physiology, cardiac output (CO), also known as heart output and often denoted by the symbols , , or , is the volumetric flow rate of the heart's pumping output: that is, the volume of blood being pumped by a single ventricle of the heart, per unit time. Cardiac output (CO) is the product of the heart rate (HR), i.e. the number of heartbeats per minute (bpm), and the stroke volume (SV), which is the volume of blood pumped from the left ventricle per beat; thus giving the formula:

A ventricle is one of two large chambers located toward the bottom of the heart that collect and expel blood towards the peripheral beds within the body and lungs. The blood pumped by a ventricle is supplied by an atrium, an adjacent chamber in the upper heart that is smaller than a ventricle. Interventricular means between the ventricles, while intraventricular means within one ventricle.
An ejection fraction (EF) is the volumetric fraction of fluid ejected from a chamber with each contraction. It can refer to the cardiac atrium, ventricle, gall bladder, or leg veins, although if unspecified it usually refers to the left ventricle of the heart. EF is widely used as a measure of the pumping efficiency of the heart and is used to classify heart failure types. It is also used as an indicator of the severity of heart failure, although it has recognized limitations.
In cardiovascular physiology, end-diastolic volume (EDV) is the volume of blood in the right or left ventricle at end of filling in diastole which is amount of blood present in ventricle at the end of diastole. Because greater EDVs cause greater distention of the ventricle, EDV is often used synonymously with preload, which refers to the length of the sarcomeres in cardiac muscle prior to contraction (systole). An increase in EDV increases the preload on the heart and, through the Frank-Starling mechanism of the heart, increases the amount of blood ejected from the ventricle during systole.
End-systolic volume (ESV) is the volume of blood in a ventricle at the end of contraction, or systole, and the beginning of filling, or diastole.

Afterload is the pressure that the heart must work against to eject blood during systole. Afterload is proportional to the average arterial pressure. As aortic and pulmonary pressures increase, the afterload increases on the left and right ventricles respectively. Afterload changes to adapt to the continually changing demands on an animal's cardiovascular system. Afterload is proportional to mean systolic blood pressure and is measured in millimeters of mercury.

Diastole is the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood. The contrasting phase is systole when the heart chambers are contracting. Atrial diastole is the relaxing of the atria, and ventricular diastole the relaxing of the ventricles.

Aortic regurgitation (AR), also known as aortic insufficiency (AI), is the leaking of the aortic valve of the heart that causes blood to flow in the reverse direction during ventricular diastole, from the aorta into the left ventricle. As a consequence, the cardiac muscle is forced to work harder than normal.
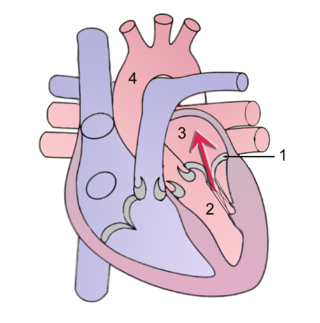
Mitral regurgitation (MR), also known as mitral insufficiency or mitral incompetence, is a form of valvular heart disease in which the mitral valve is insufficient and does not close properly when the heart pumps out blood. It is the abnormal leaking of blood backwards – regurgitation from the left ventricle, through the mitral valve, into the left atrium, when the left ventricle contracts. Mitral regurgitation is the most common form of valvular heart disease.

In cardiac physiology, preload is the amount of sarcomere stretch experienced by cardiac muscle cells, called cardiomyocytes, at the end of ventricular filling during diastole. Preload is directly related to ventricular filling. As the relaxed ventricle fills during diastole, the walls are stretched and the length of sarcomeres increases. Sarcomere length can be approximated by the volume of the ventricle because each shape has a conserved surface-area-to-volume ratio. This is useful clinically because measuring the sarcomere length is destructive to heart tissue. It requires cutting out a piece of cardiac muscle to look at the sarcomeres under a microscope. It is currently not possible to directly measure preload in the beating heart of a living animal. Preload is estimated from end-diastolic ventricular pressure and is measured in millimeters of mercury (mmHg).

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.
Pulsus paradoxus, also paradoxic pulse or paradoxical pulse, is an abnormally large decrease in stroke volume, systolic blood pressure and pulse wave amplitude during inspiration. Pulsus paradoxus is not related to pulse rate or heart rate, and it is not a paradoxical rise in systolic pressure. Normally, blood pressure drops less precipitously than 10 mmHg during inhalation. Pulsus paradoxus is a sign that is indicative of several conditions, most commonly pericardial effusion.
Cardiovascular physiology is the study of the cardiovascular system, specifically addressing the physiology of the heart ("cardio") and blood vessels ("vascular").
Impedance cardiography (ICG) is a non-invasive technology measuring total electrical conductivity of the thorax and its changes in time to process continuously a number of cardiodynamic parameters, such as stroke volume (SV), heart rate (HR), cardiac output (CO), ventricular ejection time (VET), pre-ejection period and used to detect the impedance changes caused by a high-frequency, low magnitude current flowing through the thorax between additional two pairs of electrodes located outside of the measured segment. The sensing electrodes also detect the ECG signal, which is used as a timing clock of the system.
Venous return is the rate of blood flow back to the heart. It normally limits cardiac output.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
A plot of a system's pressure versus volume has long been used to measure the work done by the system and its efficiency. This analysis can be applied to heat engines and pumps, including the heart. A considerable amount of information on cardiac performance can be determined from the pressure vs. volume plot. A number of methods have been determined for measuring PV-loop values experimentally.

Heart failure with preserved ejection fraction (HFpEF) is a form of heart failure in which the ejection fraction – the percentage of the volume of blood ejected from the left ventricle with each heartbeat divided by the volume of blood when the left ventricle is maximally filled – is normal, defined as greater than 50%; this may be measured by echocardiography or cardiac catheterization. Approximately half of people with heart failure have preserved ejection fraction, while the other half have a reduction in ejection fraction, called heart failure with reduced ejection fraction (HFrEF).
The main pathophysiology of heart failure is a reduction in the efficiency of the heart muscle, through damage or overloading. As such, it can be caused by a wide number of conditions, including myocardial infarction, hypertension and cardiac amyloidosis. Over time these increases in workload will produce changes to the heart itself: