
The heart is a muscular organ found in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest, called the mediastinum.

Systole is the part of the cardiac cycle during which some chambers of the heart contract after refilling with blood.

A ventricle is one of two large chambers located toward the bottom of the heart that collect and expel blood towards the peripheral beds within the body and lungs. The blood pumped by a ventricle is supplied by an atrium, an adjacent chamber in the upper heart that is smaller than a ventricle. Interventricular means between the ventricles, while intraventricular means within one ventricle.
In cardiovascular physiology, stroke volume (SV) is the volume of blood pumped from the ventricle per beat. Stroke volume is calculated using measurements of ventricle volumes from an echocardiogram and subtracting the volume of the blood in the ventricle at the end of a beat from the volume of blood just prior to the beat. The term stroke volume can apply to each of the two ventricles of the heart, although when not explicitly stated it refers to the left ventricle and should therefore be referred to as left stroke volume (LSV). The stroke volumes for each ventricle are generally equal, both being approximately 90 mL in a healthy 70-kg man. Any persistent difference between the two stroke volumes, no matter how small, would inevitably lead to venous congestion of and/or shunt between the systemic and the pulmonary circulation.
In cardiovascular physiology, end-diastolic volume (EDV) is the volume of blood in the right or left ventricle at end of filling in diastole which is amount of blood present in ventricle at the end of diastole. Because greater EDVs cause greater distention of the ventricle, EDV is often used synonymously with preload, which refers to the length of the sarcomeres in cardiac muscle prior to contraction (systole). An increase in EDV increases the preload on the heart and, through the Frank-Starling mechanism of the heart, increases the amount of blood ejected from the ventricle during systole.

The Frank–Starling law of the heart represents the relationship between stroke volume and end diastolic volume. The law states that the stroke volume of the heart increases in response to an increase in the volume of blood in the ventricles, before contraction, when all other factors remain constant. As a larger volume of blood flows into the ventricle, the blood stretches cardiac muscle, leading to an increase in the force of contraction. The Frank-Starling mechanism allows the cardiac output to be synchronized with the venous return, arterial blood supply and humoral length, without depending upon external regulation to make alterations. The physiological importance of the mechanism lies mainly in maintaining left and right ventricular output equality.

Afterload is the pressure that the heart must work against to eject blood during systole. Afterload is proportional to the average arterial pressure. As aortic and pulmonary pressures increase, the afterload increases on the left and right ventricles respectively. Afterload changes to adapt to the continually changing demands on an animal's cardiovascular system. Afterload is proportional to mean systolic blood pressure and is measured in millimeters of mercury.

Diastole is the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood. The contrasting phase is systole when the heart chambers are contracting. Atrial diastole is the relaxing of the atria, and ventricular diastole the relaxing of the ventricles.

Mitral stenosis is a valvular heart disease characterized by the narrowing of the opening of the mitral valve of the heart. It is almost always caused by rheumatic valvular heart disease. Normally, the mitral valve is about 5 cm2 during diastole. Any decrease in area below 2 cm2 causes mitral stenosis. Early diagnosis of mitral stenosis in pregnancy is very important as the heart cannot tolerate increased cardiac output demand as in the case of exercise and pregnancy. Atrial fibrillation is a common complication of resulting left atrial enlargement, which can lead to systemic thromboembolic complications such as stroke.

The jugular venous pressure is the indirectly observed pressure over the venous system via visualization of the internal jugular vein. It can be useful in the differentiation of different forms of heart and lung disease. Classically three upward deflections and two downward deflections have been described.

The atrium is one of the two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular mitral and tricuspid heart valves.

A pulmonary artery catheter (PAC), also known as a Swan-Ganz catheter or right heart catheter, is a balloon-tipped catheter that is inserted into a pulmonary artery in a procedure known as pulmonary artery catheterization or right heart catheterization. Pulmonary artery catheterization is a useful measure of the overall function of the heart particularly in those with complications from heart failure, heart attack, arrythmias or pulmonary embolism. It is also a good measure for those needing intravenous fluid therapy, for instance post heart surgery, shock, and severe burns. The procedure can also be used to measure pressures in the heart chambers.

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.
Pulsus paradoxus, also paradoxic pulse or paradoxical pulse, is an abnormally large decrease in stroke volume, systolic blood pressure and pulse wave amplitude during inspiration. Pulsus paradoxus is not related to pulse rate or heart rate, and it is not a paradoxical rise in systolic pressure. Normally, blood pressure drops less precipitously than 10 mmHg during inhalation. Pulsus paradoxus is a sign that is indicative of several conditions, most commonly pericardial effusion.
Venous return is the rate of blood flow back to the heart. It normally limits cardiac output.
The E/A ratio is a marker of the function of the left ventricle of the heart. It represents the ratio of peak velocity blood flow from left ventricular relaxation in early diastole to peak velocity flow in late diastole caused by atrial contraction. It is calculated using Doppler echocardiography, an ultrasound-based cardiac imaging modality. Abnormalities in the E/A ratio suggest that the left ventricle, which pumps blood into the systemic circulation, cannot fill with blood properly in the period between contractions. This phenomenon is referred to as diastolic dysfunction and can eventually lead to the symptoms of heart failure.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
A plot of a system's pressure versus volume has long been used to measure the work done by the system and its efficiency. This analysis can be applied to heat engines and pumps, including the heart. A considerable amount of information on cardiac performance can be determined from the pressure vs. volume plot. A number of methods have been determined for measuring PV-loop values experimentally.

Heart failure with preserved ejection fraction (HFpEF) is a form of heart failure in which the ejection fraction – the percentage of the volume of blood ejected from the left ventricle with each heartbeat divided by the volume of blood when the left ventricle is maximally filled – is normal, defined as greater than 50%; this may be measured by echocardiography or cardiac catheterization. Approximately half of people with heart failure have preserved ejection fraction, while the other half have a reduction in ejection fraction, called heart failure with reduced ejection fraction (HFrEF).
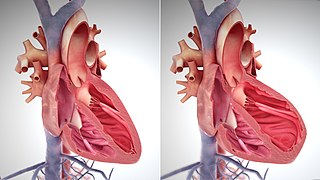
The main pathophysiology of heart failure is a reduction in the efficiency of the heart muscle, through damage or overloading. As such, it can be caused by a wide number of conditions, including myocardial infarction, hypertension and cardiac amyloidosis. Over time these increases in workload will produce changes to the heart itself:












