
The heart is a muscular organ found in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest, called the mediastinum.
Baroreceptors are sensors located in the carotid sinus and in the aortic arch. They sense the blood pressure and relay the information to the brain, so that a proper blood pressure can be maintained.

Mitral stenosis is a valvular heart disease characterized by the narrowing of the opening of the mitral valve of the heart. It is almost always caused by rheumatic valvular heart disease. Normally, the mitral valve is about 5 cm2 during diastole. Any decrease in area below 2 cm2 causes mitral stenosis. Early diagnosis of mitral stenosis in pregnancy is very important as the heart cannot tolerate increased cardiac output demand as in the case of exercise and pregnancy. Atrial fibrillation is a common complication of resulting left atrial enlargement, which can lead to systemic thromboembolic complications such as stroke.

The jugular venous pressure is the indirectly observed pressure over the venous system via visualization of the internal jugular vein. It can be useful in the differentiation of different forms of heart and lung disease. Classically three upward deflections and two downward deflections have been described.

Cardiac catheterization is the insertion of a catheter into a chamber or vessel of the heart. This is done both for diagnostic and interventional purposes.

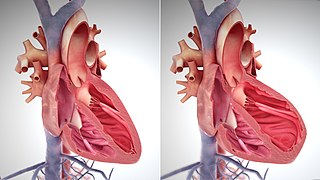
The atrium is one of the two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular mitral and tricuspid heart valves.

A pulmonary artery catheter (PAC), also known as a Swan-Ganz catheter or right heart catheter, is a balloon-tipped catheter that is inserted into a pulmonary artery in a procedure known as pulmonary artery catheterization or right heart catheterization. Pulmonary artery catheterization is a useful measure of the overall function of the heart particularly in those with complications from heart failure, heart attack, arrythmias or pulmonary embolism. It is also a good measure for those needing intravenous fluid therapy, for instance post heart surgery, shock, and severe burns. The procedure can also be used to measure pressures in the heart chambers.

In cardiac physiology, preload is the amount of sarcomere stretch experienced by cardiac muscle cells, called cardiomyocytes, at the end of ventricular filling during diastole. Preload is directly related to ventricular filling. As the relaxed ventricle fills during diastole, the walls are stretched and the length of sarcomeres increases. Sarcomere length can be approximated by the volume of the ventricle because each shape has a conserved surface-area-to-volume ratio. This is useful clinically because measuring the sarcomere length is destructive to heart tissue. It requires cutting out a piece of cardiac muscle to look at the sarcomeres under a microscope. It is currently not possible to directly measure preload in the beating heart of a living animal. Preload is estimated from end-diastolic ventricular pressure and is measured in millimeters of mercury (mmHg).

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.
Pulsus paradoxus, also paradoxic pulse or paradoxical pulse, is an abnormally large decrease in stroke volume, systolic blood pressure and pulse wave amplitude during inspiration. Pulsus paradoxus is not related to pulse rate or heart rate, and it is not a paradoxical rise in systolic pressure. Normally, blood pressure drops less precipitously than 10 mmHg during inhalation. Pulsus paradoxus is a sign that is indicative of several conditions, most commonly pericardial effusion.

The pulmonary wedge pressure (PWP) is the pressure measured by wedging a pulmonary artery catheter with an inflated balloon into a small pulmonary arterial branch. It estimates the left atrial pressure.
Venous return is the rate of blood flow back to the heart. It normally limits cardiac output.

Right atrial pressure (RAP) is the blood pressure in the right atrium of the heart. RAP reflects the amount of blood returning to the heart and the ability of the heart to pump the blood into the arterial system. RAP is often nearly identical to central venous pressure (CVP), although the two terms are not identical, as a pressure differential can sometimes exist between the venae cavae and the right atrium. CVP and RAP can differ when venous tone is altered. This can be graphically depicted as changes in the slope of the venous return plotted against right atrial pressure.

A split S2 is a finding upon auscultation of the S2 heart sound.

Volume overload refers to the state of one of the chambers of the heart in which too large a volume of blood exists within it for it to function efficiently. Ventricular volume overload is approximately equivalent to an excessively high preload. It is a cause of cardiac failure.
Low pressure baroreceptors are baroreceptors that relay information derived from blood pressure within the autonomic nervous system. They are stimulated by stretching of the vessel wall. They are located in large systemic veins and in the walls of the atria of the heart, and pulmonary vasculature. Low pressure baroreceptors are also referred to as volume receptors and cardiopulmonary baroreceptors.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
A plot of a system's pressure versus volume has long been used to measure the work done by the system and its efficiency. This analysis can be applied to heat engines and pumps, including the heart. A considerable amount of information on cardiac performance can be determined from the pressure vs. volume plot. A number of methods have been determined for measuring PV-loop values experimentally.
quantium Medical Cardiac Output (qCO) uses impedance cardiography in a simple, continuous, and non-invasive way to estimate the cardiac output (CO) and other hemodynamic parameters such as the stroke volume (SV) and cardiac index (CI). The CO estimated by the qCO monitor is referred to as the "qCO". The impedance plethysmography allows determining changes in volume of the body tissues based on the measurement of the electric impedance at the body surface.
The main pathophysiology of heart failure is a reduction in the efficiency of the heart muscle, through damage or overloading. As such, it can be caused by a wide number of conditions, including myocardial infarction, hypertension and cardiac amyloidosis. Over time these increases in workload will produce changes to the heart itself:












