Related Research Articles

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of excitable cells, which include animal cells like neurons and muscle cells, as well as some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.

The contraction of cardiac muscle in all animals is initiated by electrical impulses known as action potentials that in the heart are known as cardiac action potentials. The rate at which these impulses fire controls the rate of cardiac contraction, that is, the heart rate. The cells that create these rhythmic impulses, setting the pace for blood pumping, are called pacemaker cells, and they directly control the heart rate. They make up the cardiac pacemaker, that is, the natural pacemaker of the heart. In most humans, the highest concentration of pacemaker cells is in the sinoatrial (SA) node, the natural and primary pacemaker, and the resultant rhythm is a sinus rhythm.

Heart rate is the frequency of the heartbeat measured by the number of contractions of the heart per minute. The heart rate varies according to the body's physical needs, including the need to absorb oxygen and excrete carbon dioxide. It is also modulated by numerous factors, including genetics, physical fitness, stress or psychological status, diet, drugs, hormonal status, environment, and disease/illness, as well as the interaction between these factors. It is usually equal or close to the pulse rate measured at any peripheral point.

Hypocalcemia is a medical condition characterized by low calcium levels in the blood serum. The normal range of blood calcium is typically between 2.1–2.6 mmol/L, while levels less than 2.1 mmol/L are defined as hypocalcemic. Mildly low levels that develop slowly often have no symptoms. Otherwise symptoms may include numbness, muscle spasms, seizures, confusion, or in extreme cases cardiac arrest.

In physiology, a stimulus is a detectable change in the physical or chemical structure of an organism's internal or external environment. The ability of an organism or organ to detect external stimuli, so that an appropriate reaction can be made, is called sensitivity (excitability). Sensory receptors can receive information from outside the body, as in touch receptors found in the skin or light receptors in the eye, as well as from inside the body, as in chemoreceptors and mechanoreceptors. When a stimulus is detected by a sensory receptor, it can elicit a reflex via stimulus transduction. An internal stimulus is often the first component of a homeostatic control system. External stimuli are capable of producing systemic responses throughout the body, as in the fight-or-flight response. In order for a stimulus to be detected with high probability, its level of strength must exceed the absolute threshold; if a signal does reach threshold, the information is transmitted to the central nervous system (CNS), where it is integrated and a decision on how to react is made. Although stimuli commonly cause the body to respond, it is the CNS that finally determines whether a signal causes a reaction or not.

The sinoatrial node is an oval shaped region of special cardiac muscle in the upper back wall of the right atrium made up of cells known as pacemaker cells. The sinus node is approximately 15 mm long, 3 mm wide, and 1 mm thick, located directly below and to the side of the superior vena cava.

In electrophysiology, the threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential. In neuroscience, threshold potentials are necessary to regulate and propagate signaling in both the central nervous system (CNS) and the peripheral nervous system (PNS).
An inotrope or inotropic is a drug or any substance that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.
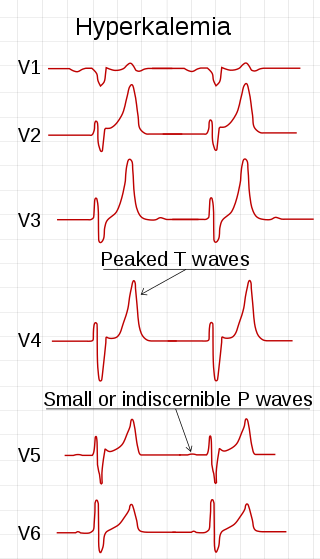
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Unlike the action potential in skeletal muscle cells, the cardiac action potential is not initiated by nervous activity. Instead, it arises from a group of specialized cells known as pacemaker cells, that have automatic action potential generation capability. In healthy hearts, these cells form the cardiac pacemaker and are found in the sinoatrial node in the right atrium. They produce roughly 60–100 action potentials every minute. The action potential passes along the cell membrane causing the cell to contract, therefore the activity of the sinoatrial node results in a resting heart rate of roughly 60–100 beats per minute. All cardiac muscle cells are electrically linked to one another, by intercalated discs which allow the action potential to pass from one cell to the next. This means that all atrial cells can contract together, and then all ventricular cells.
Hyperkalemic periodic paralysis is an inherited autosomal dominant disorder that affects sodium channels in muscle cells and the ability to regulate potassium levels in the blood. It is characterized by muscle hyperexcitability or weakness which, exacerbated by potassium, heat or cold, can lead to uncontrolled shaking followed by paralysis. Onset usually occurs in early childhood, but it still occurs with adults.
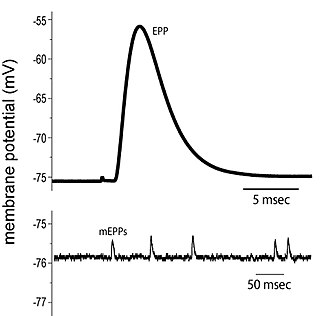
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
Myocardial contractility represents the innate ability of the heart muscle (cardiac muscle or myocardium) to contract. It is the maximum attainable value for the force of contraction of a given heart. The ability to produce changes in force during contraction result from incremental degrees of binding between different types of tissue, that is, between filaments of myosin (thick) and actin (thin) tissue. The degree of binding depends upon the concentration of calcium ions in the cell. Within an in vivo intact heart, the action/response of the sympathetic nervous system is driven by precisely timed releases of a catecholamine, which is a process that determines the concentration of calcium ions in the cytosol of cardiac muscle cells. The factors causing an increase in contractility work by causing an increase in intracellular calcium ions (Ca++) during contraction.
alpha-1 (α1) adrenergic receptors are G protein-coupled receptors (GPCRs) associated with the Gq heterotrimeric G protein. α1-adrenergic receptors are subdivided into three highly homologous subtypes, i.e., α1A-, α1B-, and α1D-adrenergic receptor subtypes. There is no α1C receptor. At one time, there was a subtype known as α1C, but it was found to be identical to the previously discovered α1A receptor subtype. To avoid confusion, naming was continued with the letter D. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α1-adrenergic receptors in the central and peripheral nervous systems. The crystal structure of the α1B-adrenergic receptor subtype has been determined in complex with the inverse agonist (+)-cyclazosin.
Tetany or tetanic seizure is a medical sign consisting of the involuntary contraction of muscles, which may be caused by disorders that increase the action potential frequency of muscle cells or of the nerves that innervate them.
Sodium channel blockers are drugs which impair the conduction of sodium ions (Na+) through sodium channels.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
Goniopora toxin (GPT) is a polypeptide toxin from the marine Goniopora species coral. Two toxins from this source have been identified, one acting on sodium channels and one acting on calcium channels.
References
- ↑ Miriam Webster's Medical Dictionary and Online Medical Dictionary
- ↑ "The Kanji Foundry Press - b". Archived from the original on 2008-03-12. Retrieved 2008-05-14.
- ↑ "Bathmotropy".
- ↑ Engelmann, Th. W. (January 1897). "Ueber den myogenen Ursprung der Herzthätigkeit und über automatische Erregbarkeit als normale Eigenschaft peripherischer Nervenfasern". Pflügers Archiv (in German). 65 (11–12): 535–578. doi:10.1007/BF01795562. ISSN 1432-2013. S2CID 31891993.
- ↑ Katz AM; Smith VE (1982). "Inotropic and lusitropic abnormalities in the genesis of heart failure". Eur Heart J. 3 (Suppl D): 11–18. doi:10.1093/eurheartj/4.suppl_a.7. PMID 6220901.
- ↑ The American Journal of the Medical Sciences. J.B. Lippincott, Company. 1908. pp. 46–.
- ↑ Scientific American Medical; Dale and Federman Vol 1; 2003 Edition p. 1907 chapter 160; Disorders of Acid-Base and Potassium Balance
- 1 2 Armstrong, C.M.; Cota, Gabriel. (1999). "Calcium block of Na+ channels and its effect on closing rate". Proceedings of the National Academy of Sciences of the United States of America . 96 (7): 4154–4157. Bibcode:1999PNAS...96.4154A. doi: 10.1073/pnas.96.7.4154 . PMC 22436 . PMID 10097179.
- ↑ Kahloon, Mansha U.; Aslam, Ahmad K.; Aslam, Ahmed F.; Wilbur, Sabrina L.; Vasavada, Balendu C.; Khan, Ijaz A. (November 2005). "Hyperkalemia induced failure of atrial and ventricular pacemaker capture". International Journal of Cardiology. 105 (2): 224–226. doi:10.1016/j.ijcard.2004.11.028. PMID 16243117.
- ↑ Veldkamp, M (1 June 2001). "Norepinephrine induces action potential prolongation and early afterdepolarizations in ventricular myocytes isolated from human end-stage failing hearts". European Heart Journal. 22 (11): 955–963. doi: 10.1053/euhj.2000.2499 . PMID 11428819.
- ↑ Ebner, F; Reiter, M (June 1979). "The alteration by propranolol of the inotropic and bathmotropic effects of dihydro-ouabain on guinea-pig papillary muscle". Naunyn-Schmiedeberg's Archives of Pharmacology. 307 (2): 99–104. doi:10.1007/BF00498450. PMID 481617. S2CID 26706163.
- ↑ Hypokalemia