
Hypoxia is a condition in which the body or a region of the body is deprived of adequate oxygen supply at the tissue level. Hypoxia may be classified as either generalized, affecting the whole body, or local, affecting a region of the body. Although hypoxia is often a pathological condition, variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise.

A motor neuron is a neuron whose cell body is located in the motor cortex, brainstem or the spinal cord, and whose axon (fiber) projects to the spinal cord or outside of the spinal cord to directly or indirectly control effector organs, mainly muscles and glands. There are two types of motor neuron – upper motor neurons and lower motor neurons. Axons from upper motor neurons synapse onto interneurons in the spinal cord and occasionally directly onto lower motor neurons. The axons from the lower motor neurons are efferent nerve fibers that carry signals from the spinal cord to the effectors. Types of lower motor neurons are alpha motor neurons, beta motor neurons, and gamma motor neurons.

The autonomic nervous system (ANS), sometimes called the visceral nervous system and formerly the vegetative nervous system, is a division of the nervous system that operates internal organs, smooth muscle and glands. The autonomic nervous system is a control system that acts largely unconsciously and regulates bodily functions, such as the heart rate, its force of contraction, digestion, respiratory rate, pupillary response, urination, and sexual arousal. The fight-or-flight response, also known as the acute stress response, is set into action by the autonomic nervous system.
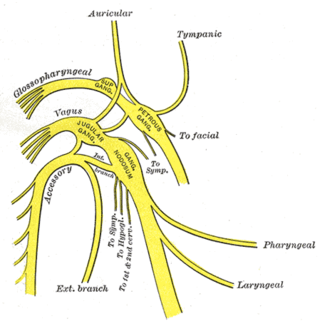
The glossopharyngeal nerve, also known as the ninth cranial nerve, cranial nerve IX, or simply CN IX, is a cranial nerve that exits the brainstem from the sides of the upper medulla, just anterior to the vagus nerve. Being a mixed nerve (sensorimotor), it carries afferent sensory and efferent motor information. The motor division of the glossopharyngeal nerve is derived from the basal plate of the embryonic medulla oblongata, whereas the sensory division originates from the cranial neural crest.
A chemoreceptor, also known as chemosensor, is a specialized sensory receptor which transduces a chemical substance to generate a biological signal. This signal may be in the form of an action potential, if the chemoreceptor is a neuron, or in the form of a neurotransmitter that can activate a nerve fiber if the chemoreceptor is a specialized cell, such as taste receptors, or an internal peripheral chemoreceptor, such as the carotid bodies. In physiology, a chemoreceptor detects changes in the normal environment, such as an increase in blood levels of carbon dioxide (hypercapnia) or a decrease in blood levels of oxygen (hypoxia), and transmits that information to the central nervous system which engages body responses to restore homeostasis.
The control of ventilation is the physiological mechanisms involved in the control of breathing, which is the movement of air into and out of the lungs. Ventilation facilitates respiration. Respiration refers to the utilization of oxygen and balancing of carbon dioxide by the body as a whole, or by individual cells in cellular respiration.

The carotid body is a small cluster of peripheral chemoreceptor cells and supporting sustentacular cells situated at the bifurcation of each common carotid artery in its tunica externa.

The solitary nucleus(SN) (nucleus of the solitary tract, nucleus solitarius, or nucleus tractus solitarii) is a series of neurons whose cell bodies form a roughly vertical column of grey matter in the medulla oblongata of the brainstem. Their axons form the bulk of the enclosed solitary tract. The solitary nucleus can be divided into different parts including dorsomedial, dorsolateral, and ventrolateral subnuclei.

A paraganglioma is a rare neuroendocrine neoplasm that may develop at various body sites. When the same type of tumor is found in the adrenal gland, they are referred to as a pheochromocytoma. They are rare tumors, with an overall estimated incidence of 1 in 300,000. There is no test that determines benign from malignant tumors; long-term follow-up is therefore recommended for all individuals with paraganglioma.

The aortic bodies are one of several small clusters of peripheral chemoreceptors located along the aortic arch. They are important in measuring partial pressures of oxygen and carbon dioxide in the blood, and blood pH.

The inferior ganglion of the glossopharyngeal nerve is a sensory ganglion. It is larger than and inferior to the superior ganglion of the glossopharyngeal nerve. It is located within the jugular foramen.

A paraganglion is a group of non-neuronal cells derived of the neural crest. They are named for being generally in close proximity to sympathetic ganglia. They are essentially of two types: (1) chromaffin or sympathetic paraganglia made of chromaffin cells and (2) nonchromaffin or parasympathetic paraganglia made of glomus cells. They are neuroendocrine cells, the former with primary endocrine functions and the latter with primary chemoreceptor functions.
Peripheral chemoreceptors are so named because they are sensory extensions of the peripheral nervous system into blood vessels where they detect changes in chemical concentrations. As transducers of patterns of variability in the surrounding environment, carotid and aortic bodies count as chemosensors in a similar way as taste buds and photoreceptors. However, because carotid and aortic bodies detect variation within the body's internal organs, they are considered interoceptors. Taste buds, olfactory bulbs, photoreceptors, and other receptors associated with the five traditional sensory modalities, by contrast, are exteroceptors in that they respond to stimuli outside the body. The body also contains proprioceptors, which respond to the amount of stretch within the organ, usually muscle, that they occupy.
Central chemoreceptors of the central nervous system, located on the ventrolateral medullary surface in the vicinity of the exit of the 9th and 10th cranial nerves, are sensitive to the pH of their environment.
The preBötzinger complex, often abbreviated as preBötC, is a functionally and anatomically specialized site in the ventral-lateral region of the lower medulla oblongata. The preBötC is part of the ventral respiratory group of respiratory related interneurons. Its foremost function is to generate the inspiratory breathing rhythm in mammals. In addition, the preBötC is widely and paucisynaptically connected to higher brain centers that regulate arousal and excitability more generally such that respiratory brain function is intimately connected with many other rhythmic and cognitive functions of the brain and central nervous system. Further, the preBötC receives mechanical sensory information from the airways that encode lung volume as well as pH, oxygen, and carbon dioxide content of circulating blood and the cerebrospinal fluid.

The respiratory center is located in the medulla oblongata and pons, in the brainstem. The respiratory center is made up of three major respiratory groups of neurons, two in the medulla and one in the pons. In the medulla they are the dorsal respiratory group, and the ventral respiratory group. In the pons, the pontine respiratory group includes two areas known as the pneumotaxic center and the apneustic center.
Hypoxic ventilatory response (HVR) is the increase in ventilation induced by hypoxia that allows the body to take in and transport lower concentrations of oxygen at higher rates. It is initially elevated in lowlanders who travel to high altitude, but reduces significantly over time as people acclimatize. In biological anthropology, HVR also refers to human adaptation to environmental stresses resulting from high altitude.
Fish are exposed to large oxygen fluctuations in their aquatic environment since the inherent properties of water can result in marked spatial and temporal differences in the concentration of oxygen. Fish respond to hypoxia with varied behavioral, physiological, and cellular responses to maintain homeostasis and organism function in an oxygen-depleted environment. The biggest challenge fish face when exposed to low oxygen conditions is maintaining metabolic energy balance, as 95% of the oxygen consumed by fish is used for ATP production releasing the chemical energy of nutrients through the mitochondrial electron transport chain. Therefore, hypoxia survival requires a coordinated response to secure more oxygen from the depleted environment and counteract the metabolic consequences of decreased ATP production at the mitochondria.
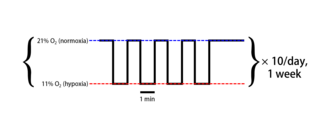
Intermittent hypoxia (also known as episodic hypoxia) is an intervention in which a person or animal undergoes alternating periods of normoxia and hypoxia. Normoxia is defined as exposure to oxygen levels normally found in Earth's atmosphere (~21% O2) and hypoxia as any oxygen levels lower than those of normoxia. Normally, exposure to hypoxia is negatively associated to physiological changes to the body, such as altitude sickness. However, when used in moderation, intermittent hypoxia may be used clinically as a means to alleviate various pathological conditions.

Cell-based therapies for Parkinson's disease include various investigational procedures which transplant specific populations of cells into the brains of people with Parkinson's disease. The investigation of cell transplantation therapies followed the discovery that the death of dopaminergic neurons in the substantia nigra pars compacta resulted in the motor symptoms of the disease. Thus, cell transplantation has focused on various dopamine producing cells throughout the body.













