Gonadotropin-releasing hormone (GnRH) is a releasing hormone responsible for the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. GnRH is a tropic peptide hormone synthesized and released from GnRH neurons within the hypothalamus. The peptide belongs to gonadotropin-releasing hormone family. It constitutes the initial step in the hypothalamic–pituitary–gonadal axis.
This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.

Goserelin, sold under the brand name Zoladex among others, is a medication which is used to suppress production of the sex hormones, particularly in the treatment of breast and prostate cancer. It is an injectable gonadotropin releasing hormone agonist.
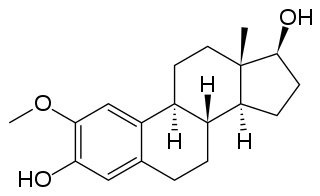
2-Methoxyestradiol is a natural metabolite of estradiol and 2-hydroxyestradiol (2-OHE2). It is specifically the 2-methyl ether of 2-hydroxyestradiol. 2-Methoxyestradiol prevents the formation of new blood vessels that tumors need in order to grow (angiogenesis), hence it is an angiogenesis inhibitor. It also acts as a vasodilator and induces apoptosis in some cancer cell lines. 2-Methoxyestradiol is derived from estradiol, although it interacts poorly with the estrogen receptors. However, it retains activity as a high-affinity agonist of the G protein-coupled estrogen receptor (GPER).

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Triptorelin, sold under the brand name Decapeptyl among others, is a medication that acts as an agonist analog of gonadotropin-releasing hormone, repressing expression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).

A gonadotropin-releasing hormone agonist is a type of medication which affects gonadotropins and sex hormones. They are used for a variety of indications including in fertility medicine and to lower sex hormone levels in the treatment of hormone-sensitive cancers such as prostate cancer and breast cancer, certain gynecological disorders like heavy periods and endometriosis, high testosterone levels in women, early puberty in children, as a part of transgender hormone therapy, and to delay puberty in transgender youth among other uses. It is also used in the suppression of spontaneous ovulation as part of controlled ovarian hyperstimulation, an essential component in IVF. GnRH agonists are given by injections into fat, as implants placed into fat, and as nasal sprays.

Gonadotropin-releasing hormone antagonists are a class of medications that antagonize the gonadotropin-releasing hormone receptor and thus the action of gonadotropin-releasing hormone (GnRH). They are used in the treatment of prostate cancer, endometriosis, uterine fibroids, female infertility in assisted reproduction, and for other indications.

Cetrorelix, or cetrorelix acetate, sold under the brand name Cetrotide, is an injectable gonadotropin-releasing hormone (GnRH) antagonist. A synthetic decapeptide, it is used in assisted reproduction to inhibit premature luteinizing hormone surges The drug works by blocking the action of GnRH upon the pituitary, thus rapidly suppressing the production and action of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, cetrorelix can be used to treat hormone-sensitive cancers of the prostate and breast and some benign gynaecological disorders. It is administered as either multiple 0.25 mg daily subcutaneous injections or as a single-dose 3 mg subcutaneous injection. The duration of the 3 mg single dose is four days; if human chorionic gonadotropin (hCG) is not administered within four days, a daily 0.25 mg dose is started and continued until hCG is administered.
Hormonal therapy in oncology is hormone therapy for cancer and is one of the major modalities of medical oncology, others being cytotoxic chemotherapy and targeted therapy (biotherapeutics). It involves the manipulation of the endocrine system through exogenous or external administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.
Antihormone therapy is a type of hormone therapy that suppresses selected hormones or their effects, in contrast with hormone replacement therapy, which encourages hormone activity.
Degarelix, sold under the brand name Firmagon among others, is a hormonal therapy used in the treatment of prostate cancer.

Bionovo was an American biotechnology company focused on the discovery and development of botanically derived treatments for women's health and cancer based in Emeryville, California. The company had multiple drug candidates in U.S. Food and Drug Administration (FDA) clinical trials- Menerba a selective estrogen receptor beta agonist for hot flashes associated with menopause Seala a selective estrogen receptor beta agonist for menopausal vaginal dryness and Bezielle for advanced breast cancer. The company has ceased activity after filing for Chapter 7 bankruptcy protection in California. Bionovo's stock is no longer listed.
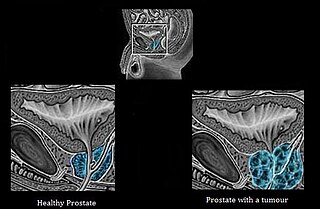
Androgen deprivation therapy (ADT), also called androgen ablation therapy or androgen suppression therapy, is an antihormone therapy whose main use is in treating prostate cancer. Prostate cancer cells usually require androgen hormones, such as testosterone, to grow. ADT reduces the levels of androgen hormones, with drugs or surgery, to prevent the prostate cancer cells from growing. The pharmaceutical approaches include antiandrogens and chemical castration.
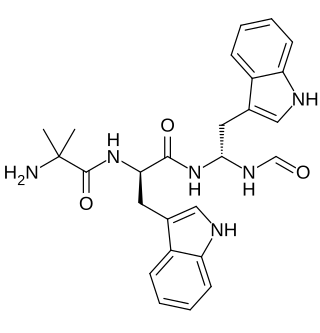
Macimorelin (INN) – or Macrilen – is a drug that was developed by Aeterna Zentaris for use in the diagnosis of adult growth hormone deficiency. Macimorelin acetate, the salt formulation, is a synthetic growth hormone secretagogue receptor agonist. It is a growth hormone secretagogue receptor agonist, causing release of growth hormone from the pituitary gland. Macimorelin acetate is described chemically as D-Tryptophanamide, 2-methylalanyl-N-[(1R)-1-(formylamino)-2-(1H-indol-3-yl)ethyl]-acetate.
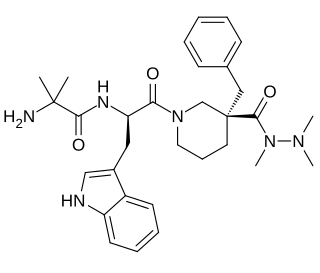
Anamorelin (INN), also known as anamorelin hydrochloride, is a non-peptide, orally-active, centrally-penetrant, selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) with appetite-enhancing and anabolic effects which is under development by Helsinn Healthcare SA for the treatment of cancer cachexia and anorexia.
A hormone-sensitive cancer, or hormone-dependent cancer, is a type of cancer that is dependent on a hormone for growth and/or survival. Examples include breast cancer, which is dependent on estrogens like estradiol, and prostate cancer, which is dependent on androgens like testosterone.

A GnRH modulator, or GnRH receptor modulator, also known as an LHRH modulator or LHRH receptor modulator, is a type of medication which modulates the GnRH receptor, the biological target of the hypothalamic hormone gonadotropin-releasing hormone. They include GnRH agonists and GnRH antagonists. These medications may be GnRH analogues like leuprorelin and cetrorelix – peptides that are structurally related to GnRH – or small-molecules like elagolix and relugolix, which are structurally distinct from and unrelated to GnRH analogues.
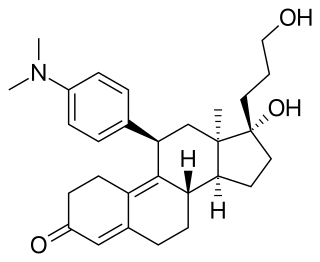
Onapristone is a synthetic and steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and described in 1984 but was never marketed. It is a silent antagonist of the progesterone receptor (PR), in contrast to the related antiprogestogen mifepristone. Moreover, compared to mifepristone, onapristone has reduced antiglucocorticoid activity, shows little antiandrogenic activity, and has 10- to 30-fold greater potency as an antiprogestogen. The medication was under development for clinical use, for instance in the treatment of breast cancer and as an endometrial contraceptive, but was discontinued during phase III clinical trials in 1995 due to findings that liver function abnormalities developed in a majority patients.
The Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) study is a large randomized controlled trial in the United Kingdom of high-dose transdermal estradiol patches versus gonadotropin-releasing hormone agonist therapy in the treatment of prostate cancer in men. It is specifically comparing three to four 100 μg/day estradiol patches against goserelin implants in approximately 2,200 men with prostate cancer. The study was started in March 2006 and is estimated for completion in August 2021, with additional reports expected in 2023 and 2024. Its objectives include comparison of survival, cardiovascular mortality and morbidity, pharmacological activity, other side effects and toxicities, and quality of life. A report on long-term cardiovascular outcomes was published in February 2021, and other reports for the study have also been published.















