 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌlɪnzəˈɡoʊlɪks/ LINZ-ə-GOH-liks |
| Trade names | Yselty |
| Other names | KLH-2109; OBE-2109 |
| Routes of administration | By mouth [1] [2] |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
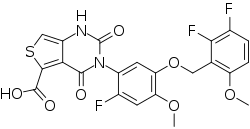
| Formula | C22H15F3N2O7S |
| Molar mass | 508.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Linzagolix, sold under the brand name Yselty, is a medication used in the treatment of uterine fibroids and endometriosis. [1] [6] [7] Linzagolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) developed by Kissei Pharmaceutical and ObsEva. [8] [9] [2]
Contents
- Medical uses
- Available forms
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Society and culture
- Legal status
- Brand names
- Availability
- References
- Further reading
- External links
In June 2022, it was approved for medical use in the European Union and in the United Kingdom. [1] [5] [10]