
Pharmacology is a branch of medicine, biology, and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

Cimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.
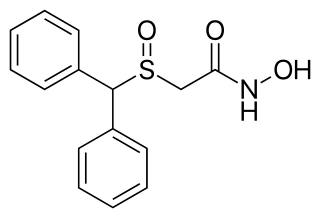
Adrafinil, sold under the brand name Olmifon, is a wakefulness-promoting medication that was formerly used in France to improve alertness, attention, wakefulness, and mood, particularly in the elderly. It was also used off-label by individuals who wished to avoid fatigue, such as night workers or others who needed to stay awake and alert for long periods of time. Additionally, the medication has been used non-medically as a nootropic.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression. Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs. It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.
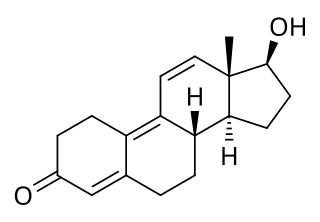
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.

Toremifene, sold under the brand name Fareston among others, is a medication which is used in the treatment of advanced breast cancer in postmenopausal women. It is taken by mouth.

Gestrinone, sold under the brand names Dimetrose and Nemestran among others, is a medication which is used in the treatment of endometriosis. It has also been used to treat other conditions such as uterine fibroids and heavy menstrual bleeding and has been investigated as a method of birth control. Gestrinone is used alone and is not formulated in combination with other medications. It is taken by mouth or in through the vagina.
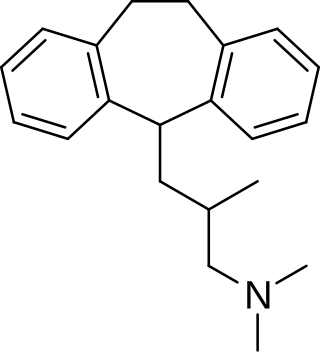
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Trilostane, sold under the brand names Modrenal and Vetoryl among others, is a medication which has been used in the treatment of Cushing's syndrome, Conn's syndrome, and postmenopausal breast cancer in humans. It was withdrawn for use in humans in the United States in the 1990s but was subsequently approved for use in veterinary medicine in the 2000s to treat Cushing's syndrome in dogs. It is taken by mouth.

Hydroxyprogesterone caproate (OHPC), sold under the brand names Proluton and Makena among others, is a progestin medication which is used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological disorders. It has also been formulated in combination with estrogens for various indications and as a form of long-lasting injectable birth control. It is not used by mouth and is instead given by injection into muscle or fat, typically once per week to once per month depending on the indication.

Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women. It is mostly no longer available. The medication is taken by mouth.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Ethynerone, also known as 17α-(2-chloroethynyl)estra-4,9-dien-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was first reported in 1961 but was never marketed. Under the developmental code name MK-665, it was studied in combination with mestranol as an oral contraceptive. Development of the drug was discontinued due to concerns surrounding toxicity findings in dogs. It is a chloroethynylated derivative of norethisterone.

Bifluranol is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that has been used as an antiandrogen in the United Kingdom in the treatment of benign prostatic hyperplasia. It is a polyfluorinated biphenyl that is related to polybrominated and polychlorinated biphenyls and diethylstilbestrol. The drug is described as a weak estrogen, and possesses about one-eighth the potency of diethylstilbestrol.
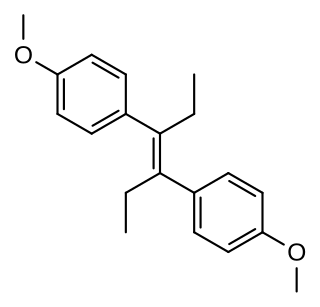
Dimestrol, also known as dianisylhexene, 4,4'-dimethoxy-α,α'-diethylstilbene, diethylstilbestrol dimethyl ether, and dimethoxydiethylstilbestrol, is a synthetic nonsteroidal estrogen of the stilbestrol group which is related to diethylstilbestrol. It has been used clinically as a hormonal therapy in cases of delayed female puberty, hypogonadism, menopausal, and postmenopausal symptoms. It is known to induce the development of female secondary sexual characteristics in the case of female delayed puberty or hypogonadism. The drug has also been used as a growth promoter in livestock.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.
Androstanolone, or stanolone, also known as dihydrotestosterone (DHT) and sold under the brand name Andractim among others, is an androgen and anabolic steroid (AAS) medication and hormone which is used mainly in the treatment of low testosterone levels in men. It is also used to treat breast development and small penis in males. It is typically given as a gel for application to the skin, but can also be used as an ester by injection into muscle.




















