
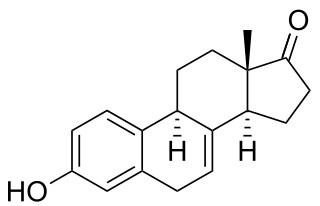
Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol.
Equilin is a naturally occurring estrogen sex hormone found in horses as well as a medication. It is one of the estrogens present in the estrogen mixtures known as conjugated estrogens and esterified estrogens. CEEs is the most commonly used form of estrogen in hormone replacement therapy (HRT) for menopausal symptoms in the United States. Estrone sulfate is the major estrogen in CEEs while equilin sulfate is the second major estrogen in the formulation, present as about 25% of the total.

Estropipate, also known as piperazine estrone sulfate and sold under the brand names Harmogen, Improvera, Ogen, Ortho-Est, and Sulestrex among others, is an estrogen medication which is used mainly in menopausal hormone therapy in the treatment of menopausal symptoms. It is a salt of estrone sulfate and piperazine, and is transformed into estrone and estradiol in the body. It is taken by mouth.

Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Paroxypropione, also known as paraoxypropiophenone, is a synthetic nonsteroidal estrogen which has been used medically as an antigonadotropin in Spain and Italy but appears to no longer be marketed. It was first synthesized in 1902. The antigonadotropic properties of the drug were discovered in 1951 and it entered clinical use shortly thereafter.

Estrapronicate (INN), also known as estradiol nicotinate propionate is an estrogen medication and estrogen ester which was never marketed. It was studied as a component of the experimental tristeroid combination drug Trophobolene, which contained nandrolone decanoate, estrapronicate, and hydroxyprogesterone heptanoate.

Doisynolic acid is a synthetic, nonsteroidal, orally active estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, the levorotatory isomer of which is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.
Estradiol stearate (E2-17-St), also known as estradiol octadecanoate and sold under the brand name Depofollan, is a naturally occurring estrogen and an estrogen ester – specifically, the C17β stearate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound is one of the components that collectively constitute lipoidal estradiol, another of which is estradiol palmitate. It is extremely lipophilic and hydrophobic. Estradiol stearate has no affinity for the estrogen receptor, requiring transformation into estradiol via esterases for its estrogenic activity. The compound does not bind to sex hormone-binding globulin or α-fetoprotein, instead being transported by lipoproteins such as high-density lipoprotein and low-density lipoprotein.

Estrone acetate is a semisynthetic, steroidal estrogen. It is an estrogen ester, specifically, an ester of estrone.

Dienestrol diacetate is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It is an ester of dienestrol.

Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate, is a natural, endogenous steroid and an estrogen ester. E2S itself is biologically inactive, but it can be converted by steroid sulfatase into estradiol, which is a potent estrogen. Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues. Estrone and E2S are the two immediate metabolic sources of estradiol. E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol. Circulating concentrations of E2S are much lower than those of E1S. High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.

Estromustine, also known as estrone 17β-3-N-bis(2-chloroethyl)carbamate or estrone–cytostatic complex, is a major active metabolite of the cystotatic antineoplastic agent and estrogen estramustine phosphate, a medication used in the treatment of prostate cancer.

Estrone cyanate, or estrone 3-O-cyanate, also known as estrocyanate, is an estrogen and an estrogen ester – specifically, the 3-O-cyanate ester of estrone – which was investigated for potential use in birth control pills but was found to be of relatively low potency and ultimately was never marketed.

Estramustine is an estrogen and cytostatic antineoplastic agent which was never marketed. It is an estrogen ester – specifically, the C3 normustine ester of estradiol – and acts in part as a prodrug of estradiol in the body. Estramustine phosphate, the C17β phosphate ester of estramustine and a prodrug of estramustine, estromustine, estradiol, and estrone, is marketed and used in the treatment of prostate cancer.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.

Estrone benzoate, or estrone 3-benzoate, is a synthetic estrogen and estrogen ester – specifically, the C3 benzoate ester of estrone – which was first reported in 1932 and was never marketed. It led to the development in 1933 of the more active estradiol benzoate, the first estradiol ester to be introduced for medical use.

Estrone methyl ether, or estrone 3-methyl ether, is a synthetic estrogen and estrogen ether – specifically, the C3 methyl ether of estrone – which was never marketed. It has been used to synthesize mestranol.















