
Benzestrol is a synthetic nonsteroidal estrogen of the stilbestrol group which was formerly used medically but has since been discontinued. The stilbestrol estrogens, the best-known of which is diethylstilbestrol (DES) were used extensively in the mid-1900s and were finally banned by the FDA due to them causing tumors in the children of women who used them.

Quinestradol, also known as quinestradiol or quinestriol, as well as estriol 3-cyclopentyl ether (E3CPE), is a synthetic estrogen and estrogen ether which is no longer marketed. It is the 3-cyclopentyl ether of estriol. The medication has been studied in the treatment of stress incontinence in elderly women, with effectiveness observed.

Hexestrol, sold under the brand name Synestrol among others, is a nonsteroidal estrogen which was previously used for estrogen replacement therapy and in the treatment of certain hormone-dependent cancers as well as gynecological disorders but is mostly no longer marketed. It has also been used in the form of esters such as hexestrol diacetate and hexestrol dipropionate. Hexestrol and its esters are taken by mouth, held under the tongue, or via injection into muscle.

Cloxestradiol (INN), also known as 17-(2,2,2-trichloroethoxy)estradiol, is a synthetic, steroidal estrogen which was never marketed. It is an analogue of estradiol with a 2,2,2-trichloroethoxy substitution. The O,O-diacetate derivative, cloxestradiol acetate, has been marketed as an estrogen.

Cloxestradiol acetate, also known as 17-(2,2,2-trichloroethoxy)estradiol O,O-diacetate, is a synthetic steroidal estrogen derived from estradiol. It is the O,O-diacetate ester of cloxestradiol, which, in contrast to cloxestradiol acetate, was never marketed.
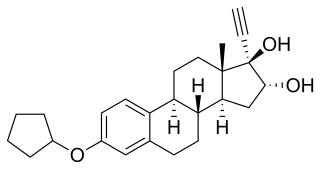
Nilestriol (INN), also known as nylestriol, is a synthetic estrogen which was patented in 1971 and is marketed in China. It is the 3-cyclopentyl ether of ethinylestriol, and is also known as ethinylestriol cyclopentyl ether (EE3CPE). Nilestriol is a prodrug of ethinylestriol, and is a more potent estrogen in comparison. It is described as a slowly-metabolized, long-acting estrogen and derivative of estriol. Nilestriol was assessed in combination with levonorgestrel for the potential treatment of postmenopausal osteoporosis, but this formulation ultimately was not marketed.

Orestrate (INN), also known as estradiol 3-propionate 17β-(1-cyclohexenyl) ether, is an estrogen medication and estrogen ester which was never marketed. It is the C3 propionate ester and C17β-(1-cyclohexenyl) ether of estradiol.

Estradiol furoate (EF), or estradiol 17β-furoate, sold under the brand name Di-Folliculine, is an estrogen medication and estrogen ester which is no longer marketed. It is the C17β furoate ester of estradiol. Estradiol benzoate has also been marketed under the brand name Di-Folliculine, and should not be confused with estradiol furoate.

Hexestrol dipropionate, or hexestrol dipropanoate, is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It is an ester of hexestrol, and has been known since at least 1931. The drug has been used in the past to inhibit lactation in women.
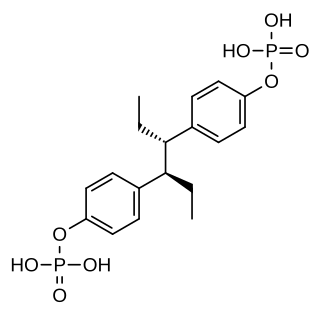
Hexestrol diphosphate is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol and used as an estrogen and antineoplastic agent in the treatment of prostate cancer. It is a water-soluble ester of hexestrol. The medication has been known since at least 1956.
Hexestrol dicaprylate, or dioctanoylhexestrol, is a synthetic, nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that is no longer marketed. It is a long-acting ester of hexestrol.

Mestilbol, also known as diethylstilbestrol monomethyl ether, is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It was developed by Wallace & Tiernan Company, patented in 1940, and introduced for medical use in the 1940s, but is now no longer marketed. Mestilbol was available both as oral tablets and in oil for intramuscular injection. The drug is gradually demethylated in the body into diethylstilbestrol and hence is a prodrug of diethylstilbestrol. Mestilbol is a highly active estrogen, although somewhat less so than diethylstilbestrol, but is longer-lasting in comparison.
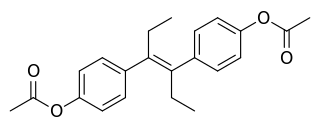
Diethylstilbestrol diacetate (DESDA) is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ester of diethylstilbestrol (DES) that was introduced for clinical use in the 1940s and was formerly marketed but is now no longer available.
Phenestrol, or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard ester of hexestrol which was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.

Estradiol mustard, also known as chlorphenacyl estradiol diester, as well as estradiol 3,17β-bis(4- phenyl)acetate, is a synthetic, steroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard-coupled estrogen ester that was never marketed. It is selectively distributed into estrogen receptor (ER)-positive tissues such as ER-expressing tumors like those seen in breast and prostate cancers. For this reason, estradiol mustard and other cytostatic-linked estrogens like estramustine phosphate have reduced toxicity relative to non-linked nitrogen mustard cytostatic antineoplastic agents. However, they may stimulate breast tumor growth due to their inherent estrogenic activity and are said to be devoid of major therapeutic efficacy in breast cancer, although estramustine phosphate has been approved for and is used in the treatment of prostate cancer.
Lipoidal estradiol (LE2) is the variety of endogenous C17β long-chain fatty acid esters of estradiol which are formed as metabolites of estradiol. Important examples of these esters include estradiol arachidonate, estradiol lineolate, estradiol oleate, estradiol palmitate, and estradiol stearate. LE2 are estrogens but do not bind to the estrogen receptor, instead acting as prohormones of estradiol. Relative to estradiol, they have far longer-lasting durations of effect due to their much slower rates of metabolism and clearance. It has been hypothesized that LE2 may serve as a store of estrogen for when estradiol levels become low. LE2 are highly lipophilic and hydrophobic and are found in highest concentrations in adipose tissue and other estrogen-sensitive tissues and in low but detectable concentrations in circulation, with none excreted in urine. They have been referred to as the "endogenous counterparts of the synthetic esters of estrogens" like estradiol valerate and estradiol cypionate.

Estrone benzoate, or estrone 3-benzoate, is a synthetic estrogen and estrogen ester – specifically, the C3 benzoate ester of estrone – which was first reported in 1932 and was never marketed. It led to the development in 1933 of the more active estradiol benzoate, the first estradiol ester to be introduced for medical use.

Ethinylestradiol benzoate, or 17α-ethynylestradiol 3-benzoate, is a synthetic estrogen and estrogen ester – specifically, the C3 benzoate ester of ethinylestradiol – which was first described in the late 1930s and was never marketed.

Estrone methyl ether, or estrone 3-methyl ether, is a synthetic estrogen and estrogen ether – specifically, the C3 methyl ether of estrone – which was never marketed. It has been used to synthesize mestranol.
















