| |
| Names | |
|---|---|
| Preferred IUPAC name N,N-Dimethyl-1,2-dithiolan-4-amine | |
| Other names NTX | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H11NS2 | |
| Molar mass | 149.27 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
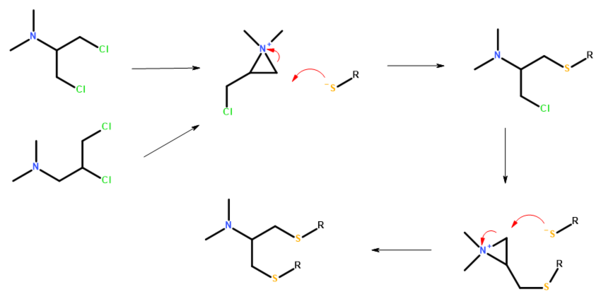
Nereistoxin is a natural product identified in 1962 as the toxic organic compound N,N-dimethyl-1,2-dithiolan-4-amine. It had first been isolated in 1934 from the marine annelid Lumbriconereis heteropoda and acts by blocking the nicotinic acetylcholine receptor. [1] [2] Researchers at Takeda in Japan investigated it as a possible insecticide. They subsequently developed a number of derivatives that were commercialised, [3] [4] including those with the ISO common names [5] bensultap, [6] cartap, [7] thiocyclam [8] and thiosultap. [9] [10]