
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934%. It is more than twice as abundant as water vapor, 23 times as abundant as carbon dioxide, and more than 500 times as abundant as neon. Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust.
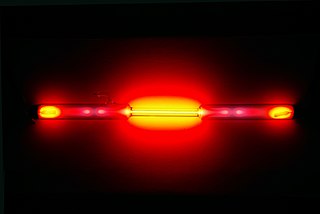
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of air.

A vacuum is space devoid of matter. The word is derived from the Latin adjective vacuus meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often discuss ideal test results that would occur in a perfect vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is considerably lower than atmospheric pressure. The Latin term in vacuo is used to describe an object that is surrounded by a vacuum.

Liquid hydrogen (H2(l)) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully.

A glovebox is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hands into the gloves and perform tasks inside the box without breaking containment. Part or all of the box is usually transparent to allow the user to see what is being manipulated. Two types of gloveboxes exist. The first allows a person to work with hazardous substances, such as radioactive materials or infectious disease agents, and the second allows manipulation of substances that must be contained within a very high purity inert atmosphere, such as argon or nitrogen. It is also possible to use a glovebox for manipulation of items in a vacuum chamber.

A relief valve or pressure relief valve (PRV) is a type of safety valve used to control or limit the pressure in a system; excessive pressure might otherwise build up and create a process upset, instrument or equipment failure, explosion, or fire.

The Schlenk line is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is connected to a vacuum pump. The inert-gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the vacuum pump by a liquid-nitrogen or dry-ice/acetone cold trap. Special stopcocks or Teflon taps allow vacuum or inert gas to be selected without the need for placing the sample on a separate line.
Air sensitivity is a term used, particularly in chemistry, to denote the reactivity of chemical compounds with some constituent of air. Most often, reactions occur with atmospheric oxygen (O2) or water vapor (H2O), although reactions with the other constituents of air such as carbon monoxide (CO), carbon dioxide (CO2), and nitrogen (N2) are also possible.
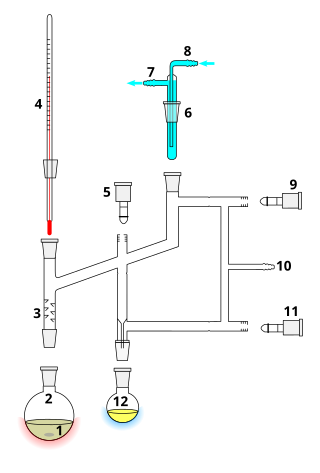
A Perkin triangle is a specialized apparatus for the distillation of air-sensitive materials. It is named after William Henry Perkin Jr., whose design was approximately triangular. The diagram shows a more modern version, in which the glass taps have been replaced with more air-tight Teflon taps.
A Schlenk flask, or Schlenk tube, is a reaction vessel typically used in air-sensitive chemistry, invented by Wilhelm Schlenk. It has a side arm fitted with a PTFE or ground glass stopcock, which allows the vessel to be evacuated or filled with gases. These flasks are often connected to Schlenk lines, which allow both operations to be done easily.

A sublimatory or sublimation apparatus is equipment, commonly laboratory glassware, for purification of compounds by selective sublimation. In principle, the operation resembles purification by distillation, except that the products do not pass through a liquid phase.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.

Cannula transfer or cannulation is a set of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. While the syringes are not the same as cannulae, the techniques remain relevant.

Nitrogen generators and stations are stationary or mobile air-to-nitrogen production complexes.

Packaging machinery is used throughout all packaging operations, involving primary packages to distribution packs. This includes many packaging processes: fabrication, cleaning, filling, sealing, combining, labeling, overwrapping, palletizing.
Gallium(I) oxide, digallium monoxide or gallium suboxide is an inorganic compound with the formula Ga2O.

The Planetary Material Sample Curation Facility (PMSCF), commonly known as the Extraterrestrial Sample Curation Center is the facility where Japan Aerospace Exploration Agency (JAXA) conducts the curation works of extraterrestrial materials retrieved by some sample-return missions. They work closely with Japan's Astromaterials Science Research Group. Its objectives include documentation, preservation, preparation, and distribution of samples. All samples collected are made available for international distribution upon request.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.















