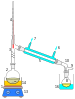
Operation
The solvent is heated to reflux. The solvent vapour travels up a distillation arm, and floods into the chamber housing the thimble of solid. The condenser ensures that any solvent vapour cools, and drips back down into the chamber housing the solid material. The chamber containing the solid material slowly fills with warm solvent. Some of the desired compound dissolves in the cold solvent. When the Soxhlet chamber is almost full, the chamber is emptied by the siphon. The solvent is returned to the distillation flask. The thimble ensures that the rapid motion of the solvent does not transport any solid material to the still pot. This cycle may be allowed to repeat many times, over hours or days.
During each cycle, a portion of the non-volatile compound dissolves in the solvent. After many cycles the desired compound is concentrated in the distillation flask. The advantage of this system is that instead of many portions of warm solvent being passed through the sample, just one batch of solvent is recycled.
After extraction the solvent is removed, typically by means of a rotary evaporator, yielding the extracted compound. The non-soluble portion of the extracted solid remains in the thimble, and is usually discarded. Like Soxhlet extractor, the Kumagawa extractor has a specific design where the thimble holder/chamber is directly suspended inside the solvent flask (having a vertical large opening) above the boiling solvent. The thimble is surrounded by hot solvent vapour and maintained at a higher temperature compared to the Soxhlet extractor, thus allowing better extraction for compounds with higher melting points such as bitumen. The removable holder/chamber is fitted with a small siphon side arm and, in the same way as for Soxhlet, a vertical condenser ensures that the solvent drips back down into the chamber which is automatically emptied at every cycle.
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
![A schematic representation of a Soxhlet extractor:
.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key>ol{margin-left:1.3em;margin-top:0}.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key>ul{margin-top:0}.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key li{page-break-inside:avoid;break-inside:avoid-column}@media(min-width:300px){.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key,.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key-wide{column-count:2}.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key-narrow{column-count:1}}@media(min-width:450px){.mw-parser-output figure[typeof="mw:File/Thumb"] .image-key-wide{column-count:3}}
Stirrer bar
Still pot (the still pot should not be overfilled and the volume of solvent in the still pot should be 3 to 4 times the volume of the Soxhlet chamber)
Distillation path
Thimble
Solid
Siphon top
Siphon exit
Expansion adapter
Condenser
Cooling water out
Cooling water in Soxhlet extractor.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e0/Soxhlet_extractor.svg/220px-Soxhlet_extractor.svg.png)